TECHNOLOGY BY SURGEONS.¹
for use after liposuction, and the
only device that is FDA-cleared for
contracting subcutaneous tissue.²


The only device that is FDA-cleared for use after liposuction, and the only device that is FDA-cleared for contracting subcutaneous tissue.²

REAL RESULTS

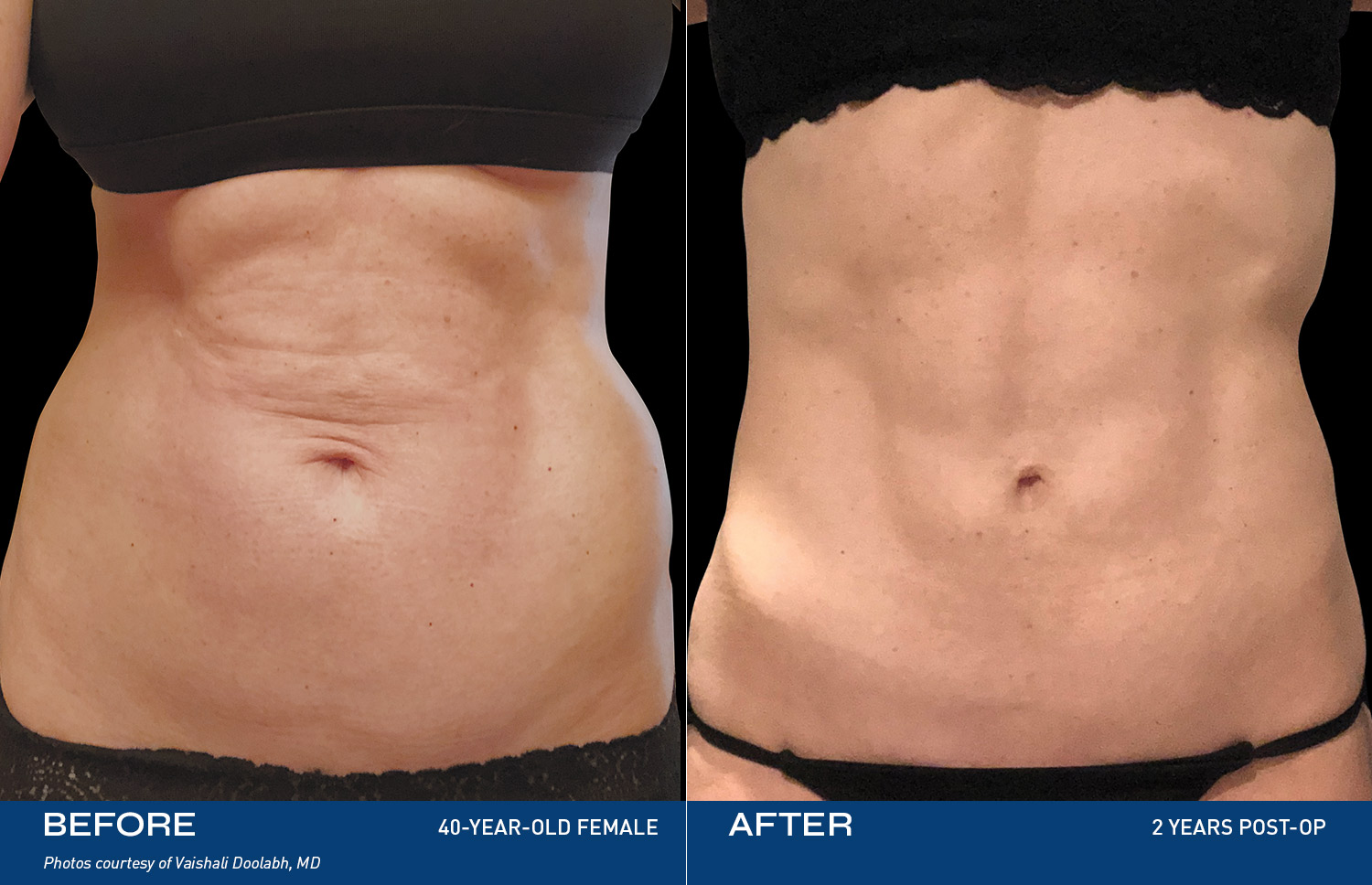
CONTOURING
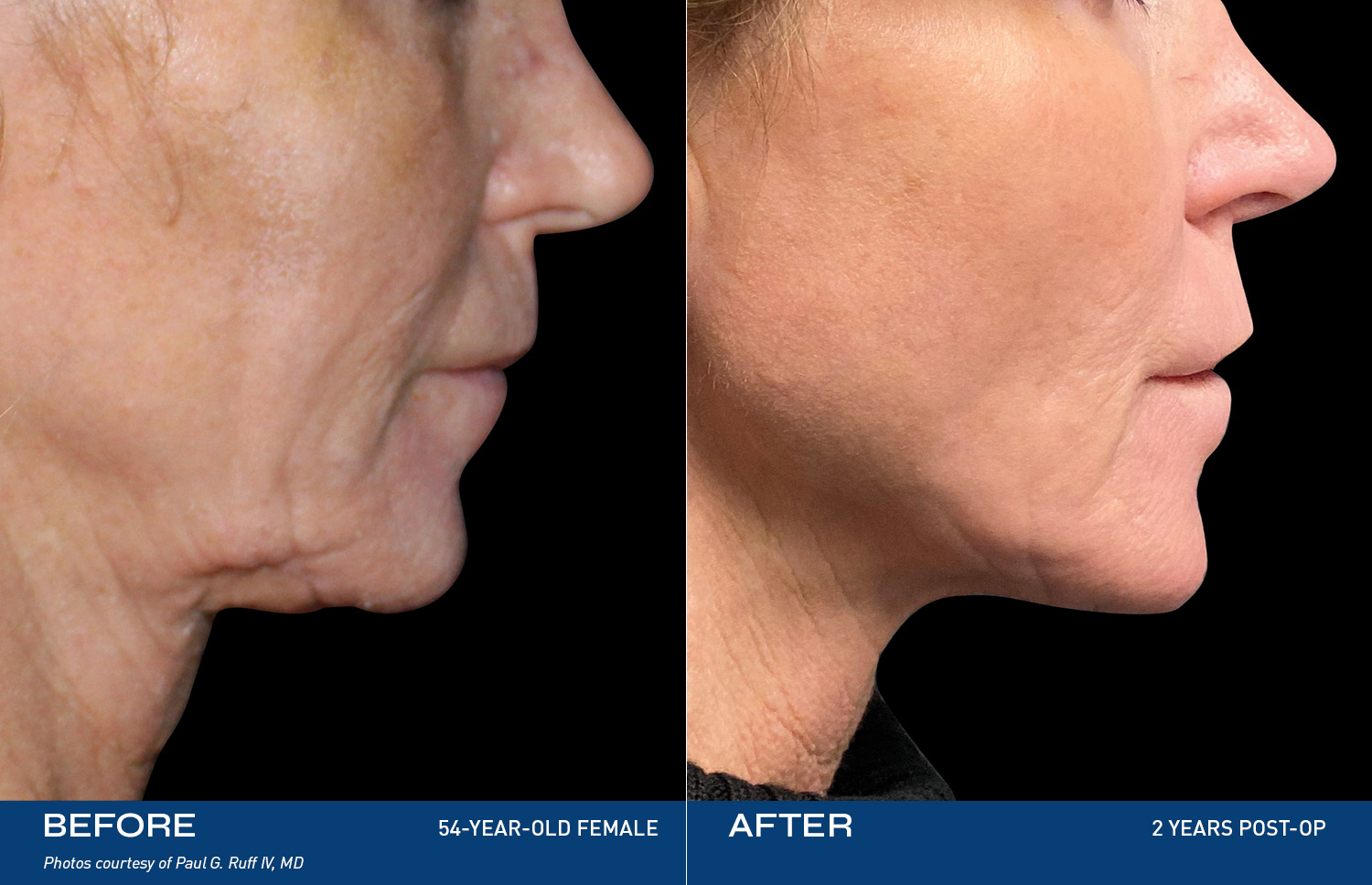
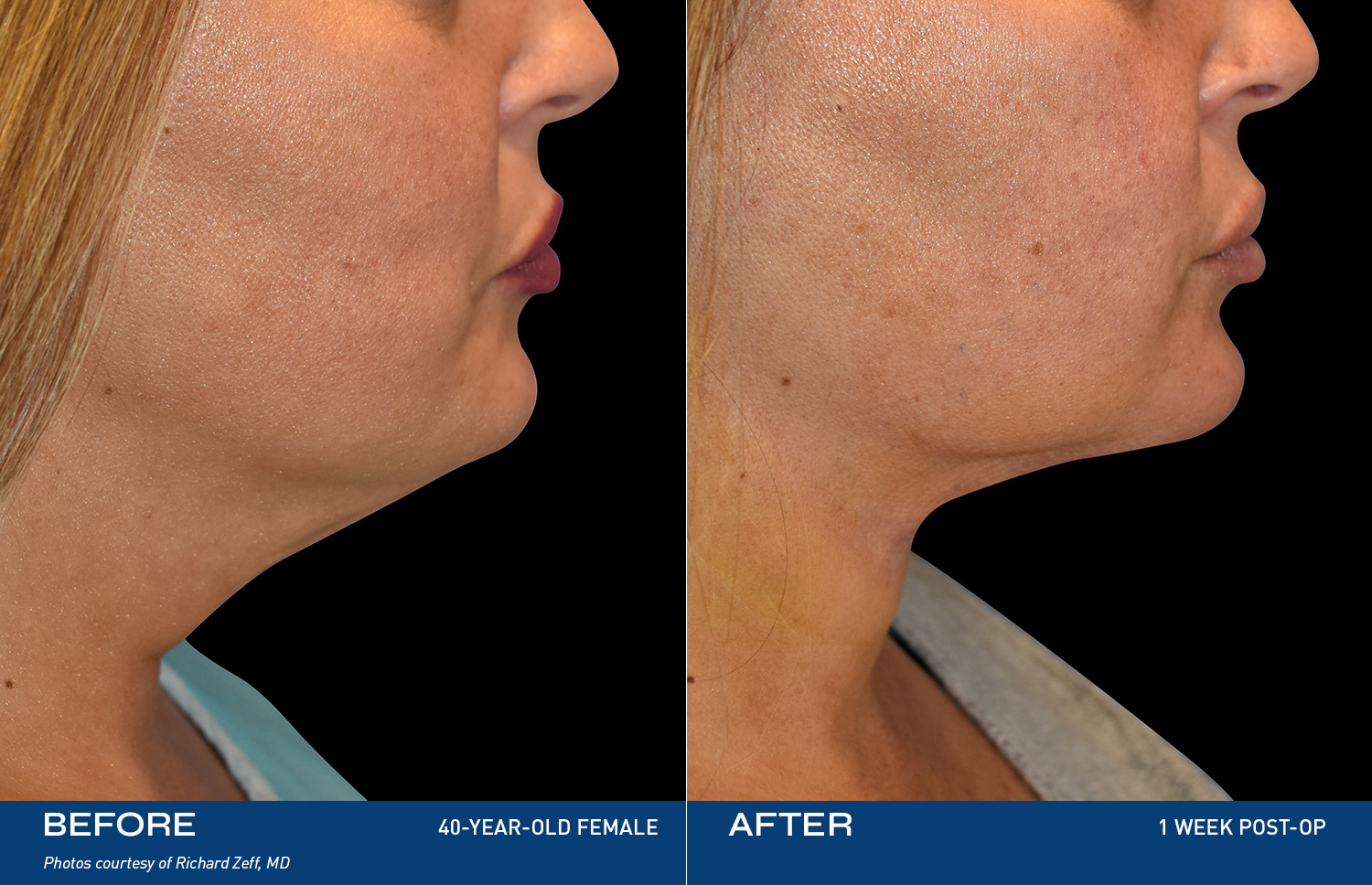
The Renuvion Body
Precise Tissue Contraction4-9
Renuvion is unique in that it allows for near instantaneous tissue contraction and directly addresses the issue of skin laxity, a key problem patients of all ages are experiencing.REAL RESULTS
BEFORE & AFTERS
"I tried so many things, but my skin was still loose. After Renuvion my neck and jawline look so smooth and it feels tight like it used to. I'm so happy with the result."
- Renuvion Patient
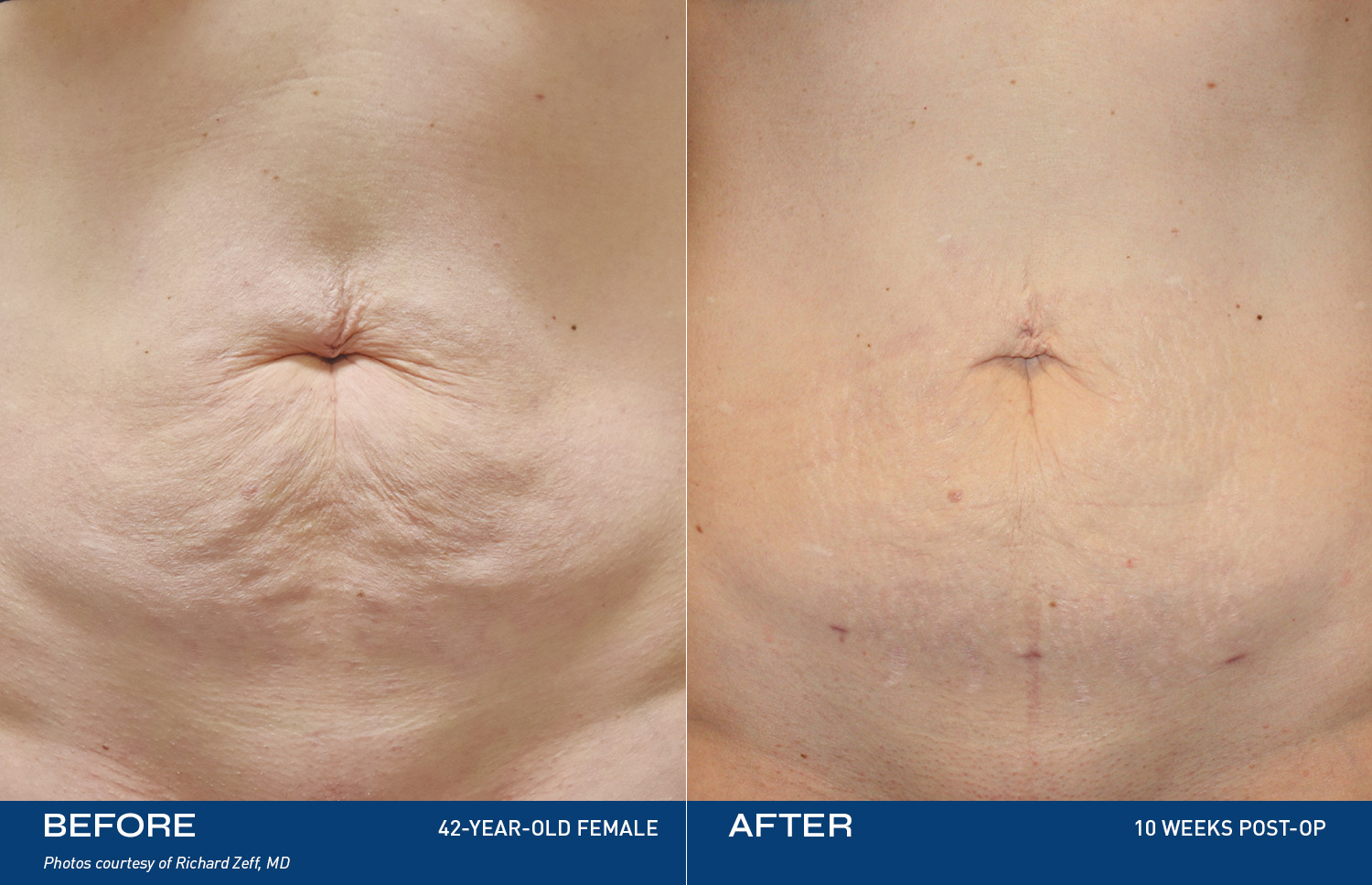
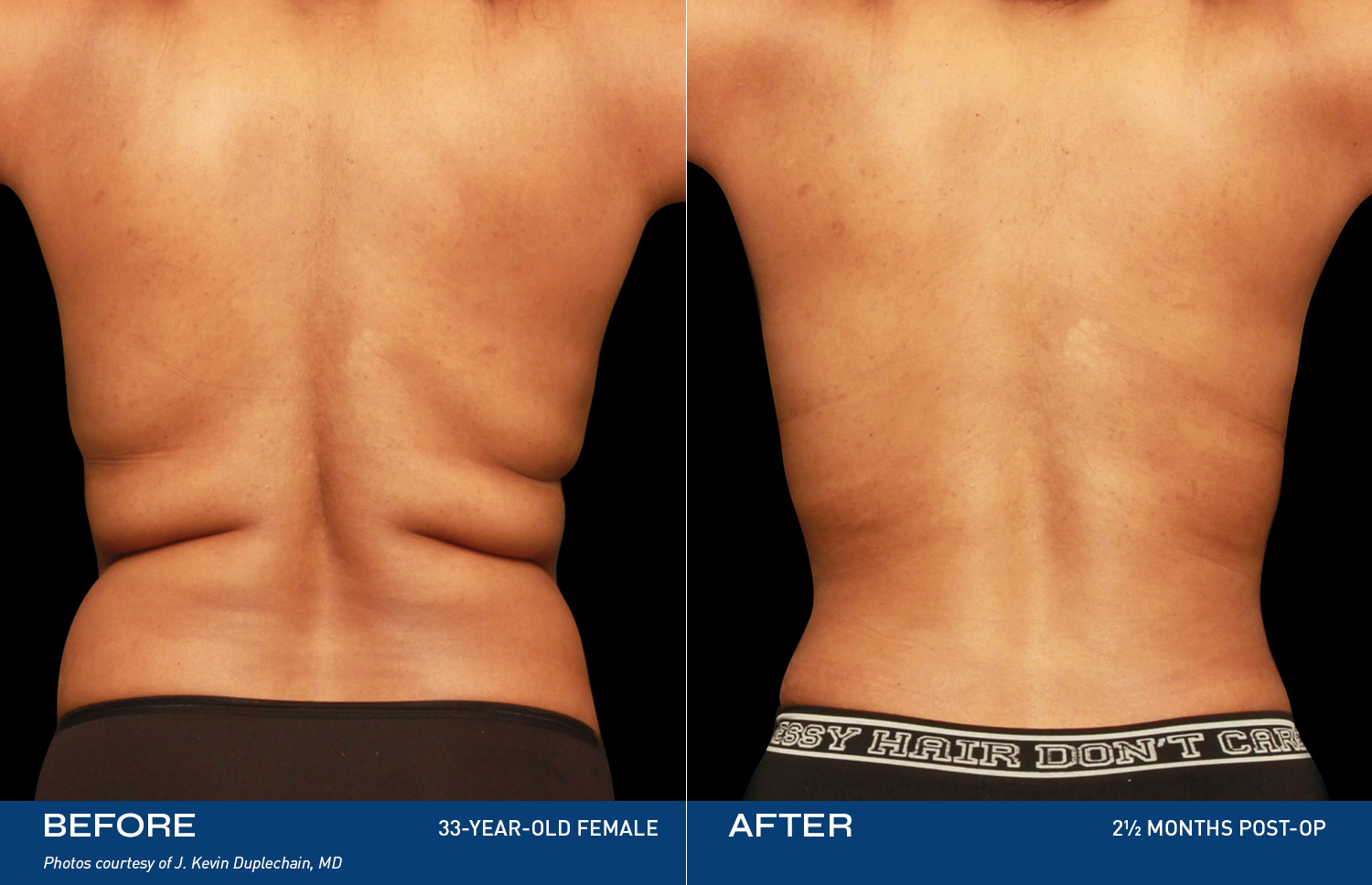
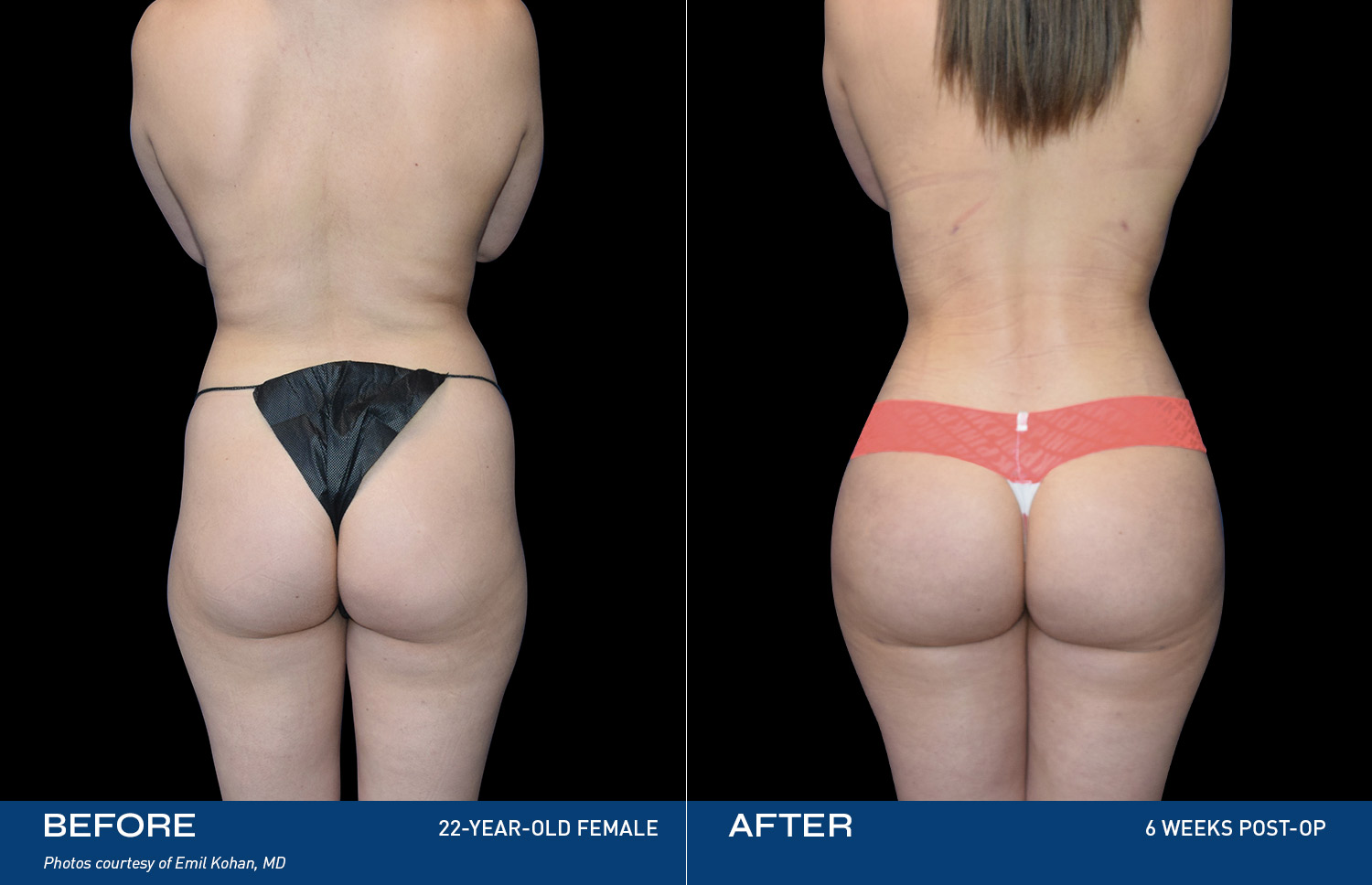
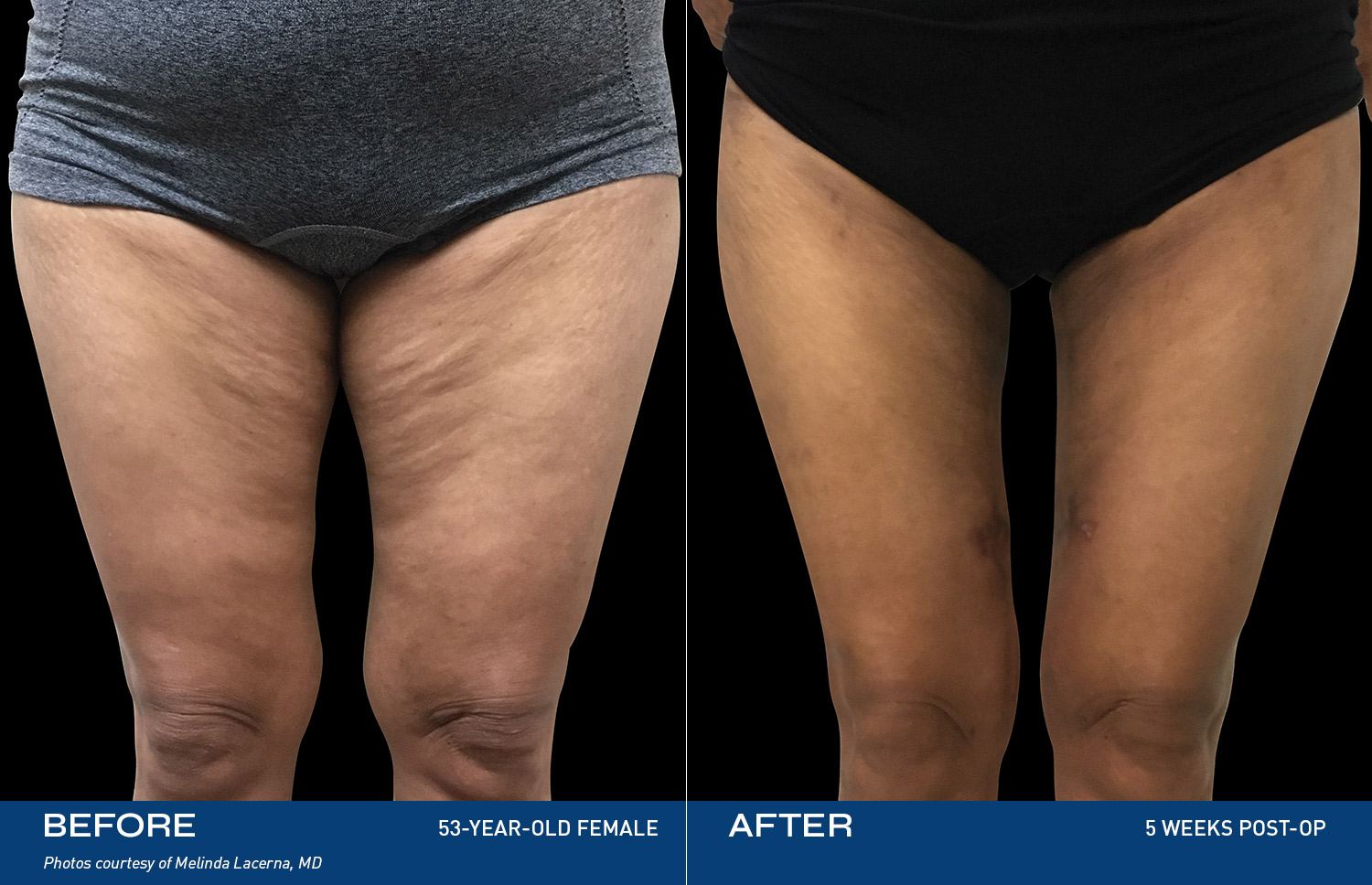
DEVICE OVERVIEW
WHAT IS RENUVION?
Renuvion is a minimally-invasive procedure that uses helium plasma and proprietary RF energy to contour and improve the appearance of loose skin. A product of years of scientific research, testing, and refinement, the Renuvion system is also an all-in-one electrosurgical unit with full monopolar and bipolar functions, allowing you to save time and space with a single capital investment.
HOW IT WORKS
Fast, Innovative Tissue Contraction
RENUVION
TECHNOLOGY
Unique Helium Plasma technology
A matter of energy
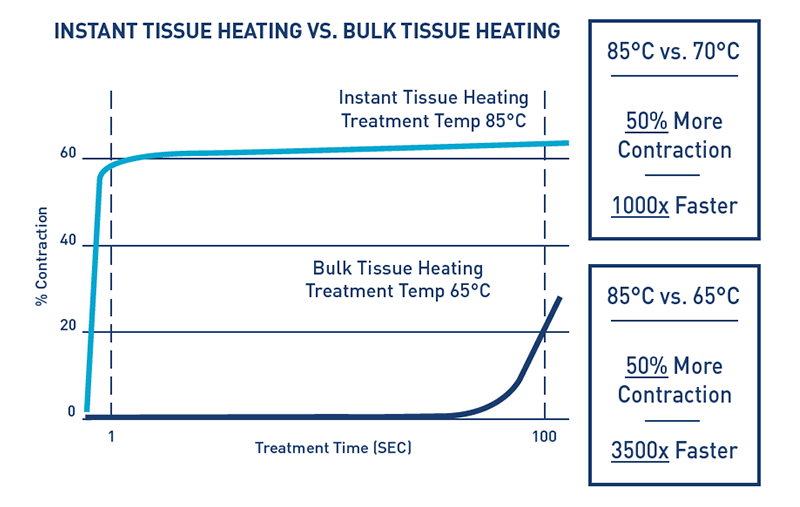
- Higher temperatures cause greater tissue contraction (figure 1)4-8
- Collagen contracts predictably when exposed to heat, and higher temperatures cause greater contraction in less time
- To be effective, subdermal tissue must be heated to a minimum of 65°C for significant tissue contraction to occur
- Standard radiofrequency devices can’t consistently heat beyond 65°C10
- 85°C is a more optimal temperature for tissue contraction, but most standard monopolar and bipolar radiofrequency devices can’t reach this temperature quickly enough without causing a concerning rise in skin temperature
- Renuvion heats to 85°C safely, allowing for optimal tissue contraction and faster procedure times4-9,10
- Renuvion heats up to 85°C just long enough to cause maximum contraction of collagen and can cool back down to baseline temperatures in less than a second (figure 2)
- The rapid heating and cooling of tissue allows for shorter application time
Safety By Design
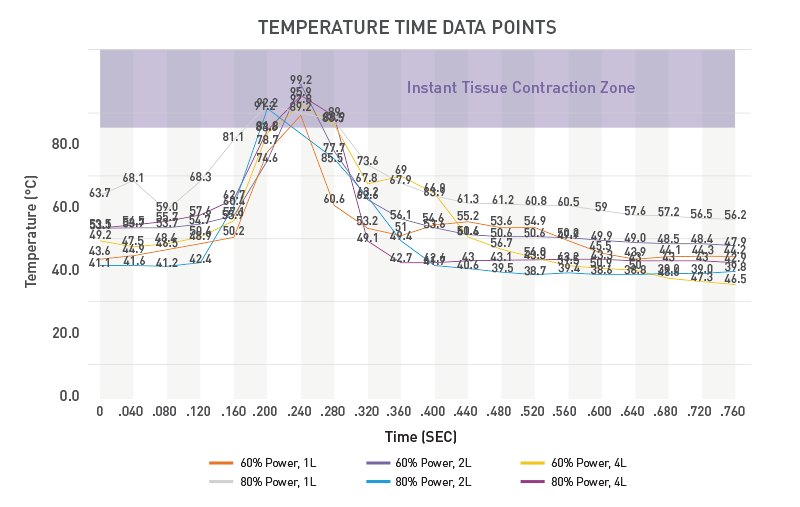
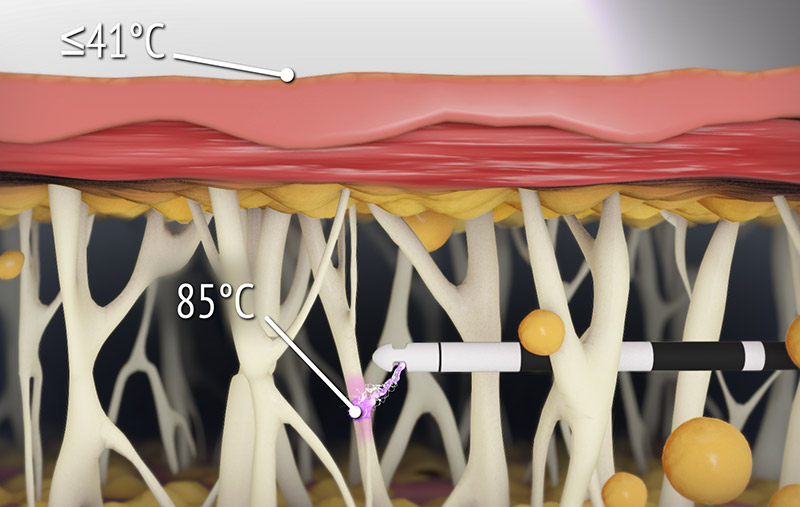
- The unique Renuvion energy— helium plasma and proprietary RF — allows for precisely controlled delivery of heat to tissue, with minimal thermal diffusion9,10
- Rapid heating with near-instantaneous cooling (figure 3) allows for shorter duration of activation, and therefore less diffusion of heat to the skin10
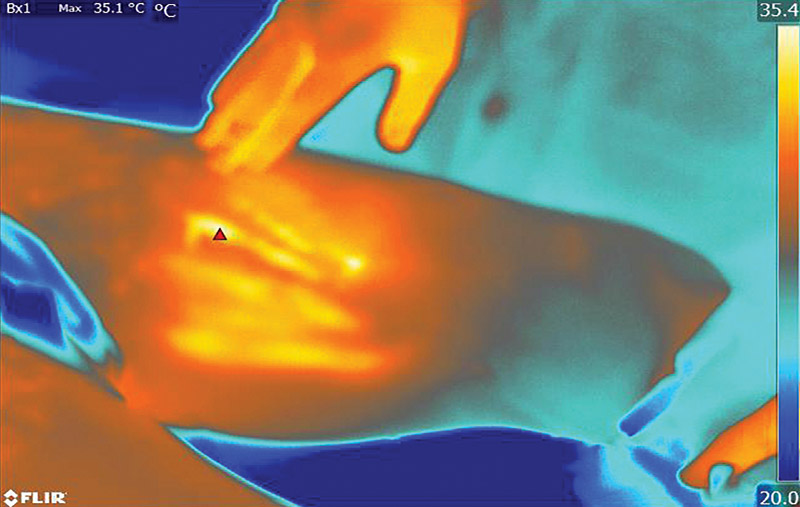
- Studies show that when using the recommended techniques, temperature at the surface of the skin does not rise by more than 4°C, making cumbersome external temperature monitoring unnecessary (figure 4)10
Built On Proven Technology
doctor
Testimonials
doctor
PAUL NASSIF
doctor
Tabasum Mir
doctor
Leif Rogers
doctor
PAUL RUFF
doctor
Alexis Delobaux
doctor
Gregory Buford
PATIENT BENEFITS
- Minimally invasive10
- Shorter Procedure11
- Less Pain11
- Shorter Recovery Time11
- Visible Results Quickly11
- Long-Term Results11
- Less Risk of Complications11*
*compared to more invasive surgery
PRACTICE BENEFITS
- Competitive Differentiator
- New Source of Revenue
- Increased Patient Leads
- Higher Patient Satisfaction12
- Consistent Outcomes11
- Industry Leading 4-Year Warranty
The Renuvion APR Handpiece is intended for the delivery of radiofrequency energy and/or helium plasma where coagulation/contraction of soft tissue is needed. Soft tissue includes subcutaneous tissue. The Renuvion APR Handpiece is intended for the coagulation of subcutaneous soft tissues following liposuction for aesthetic body contouring. The Renuvion APR Handpiece is indicated for use in subcutaneous dermatological and aesthetic procedures to improve the appearance of lax (loose) skin in the neck and submental region. The Renuvion APR Handpiece is intended for the delivery of radiofrequency energy and/or helium plasma for cutting, coagulation and ablation of soft tissue during open surgical procedures. The Renuvion APR Handpiece is intended to be used with compatible electrosurgical generators owned by Apyx Medical. Not all indications are approved in all markets; check with your local sales representative for further information.
As with any aesthetic procedure, individual results may vary. As with all energy devices there are inherent risks associated with its use. Risk associated with the use of the Renuvion APR may include: helium embolism into the surgical site due to inadvertent introduction into the venous or arterial blood supply system, unintended burns (deep or superficial), pneumothorax, temporary or permanent nerve injury, ischemia, fibrosis, infection, pain, discomfort, gas buildup resulting in temporary and transient crepitus or pain, bleeding, hematoma, seroma, subcutaneous induration, pigmentation changes, increased healing time, scarring, asymmetry and/ or unacceptable cosmetic result. Please see the instructions for use for more detailed information.
References:
- 4 out of 5 surgeons agree Renuvion is the #1 trusted body contouring technology. Results based on a 2024 Renuvion Physician survey conducted by Wakefield Research. Data on File.
- FDA 510(k) Premarket Notifications K230272 & K223262.https://www.clinicaltrials.gov/ct2/results?term=NCT04146467.
- ISAPS International Survey on Aesthetic/Cosmetic Procedures 2021. https://www.isaps.org/media/vdpdanke/isaps-global-survey_2021.pdf
- Feldman LS, et al. (eds). The SAGES Manual on the Fundamental Use of Surgical Energy (FUSE), ISBN 978‐1‐4614‐2073‐
- Chen SS, Wright NT, Humphrey JD. Heat-induced changes in the mechanics of a collagenous tissue: isothermal free shrinkage. Journal of Biomechanical Engineering 1997:109:372-378.
- McDonald MB. Conductive Keratoplasty: A Radiofrequency-based Technique for the Correction of Hyperopia. Trans Am Ophthalmol Soc 2005;103:512-536.
- Chen SS, Humphrey JD. Heat-induced changes in the mechanics of a collagenous tissue: pseudoelastic behavior at 37° C. J Biomech 1998;31:211-216.
- Wright NT, Humphrey JD. Denaturation of collagen during heating: An irreversible rate process. Annu Rev Biomed Eng; 2002;4:109-128.
- Masghati S, Pedroso J, Gutierrez M, Stockwell E, Volker W, Howard DL. Comparative Thermal Effects of J-Plasma®, Monopolar, Argon, and Laser Electrosurgery in a Porcine Tissue Model. Surgical Technology International, 2019;34:1-5. PMID: 30825320.
- Duncan DI and Roman S. Helium Plasma Subdermal Tissue Contraction Method of Action. Biomed J Sci & Tech Res 31(2)-2020. BJSTR. MS.ID.005075.
- Renuvion Physician Survey Results, MM0317.01 0422 – https://www.renuvion.org/wp-content/uploads/2022/05/renuvion-physician-survey-results-brochure_mm0317.01_050222.pdf.
- Ruff PG, Doolabh V, Zimmerman EM, Gentile RA. Safety and efficacy of helium plasma for subdermal coagulation. Dermatological Reviews. 2020;1-7. https://doi.org/10.1002/der2.34.
LATEST NEWS
AS SEEN IN