
for use after liposuction, and the
only device that is FDA-cleared for
contracting subcutaneous tissue.


for use after liposuction, and the
only device that is FDA-cleared for
contracting subcutaneous tissue.


REAL RESULTS
BOOK A DEMO
Enter your Name, Email and Zip Code below and revitalize your practice.
RENUVION
REAL RESULTS
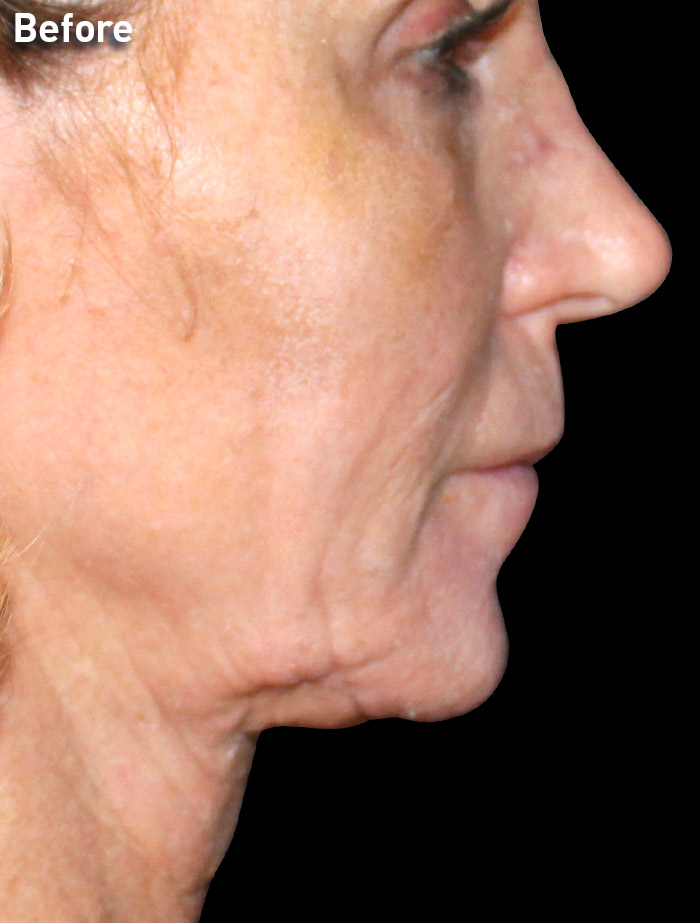
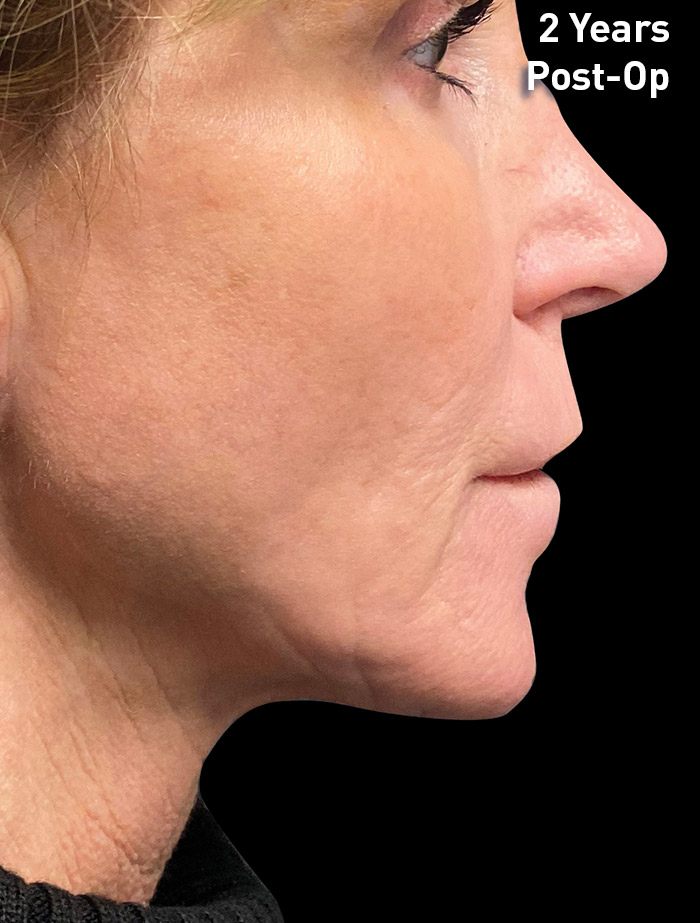
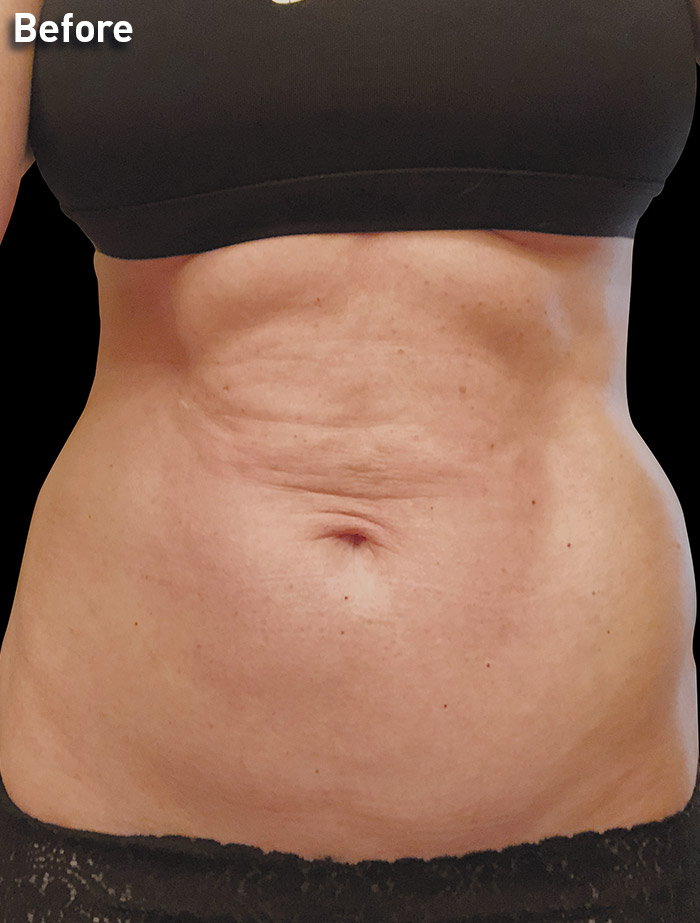
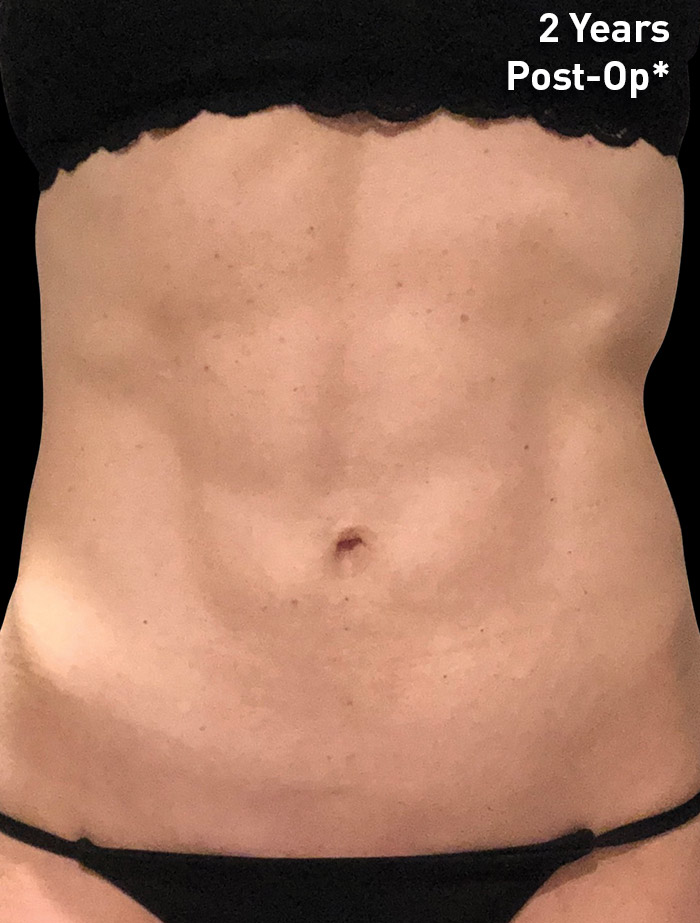
CASE 1
54-year-old female
Photos courtesy of Paul G. Ruff IV, MD
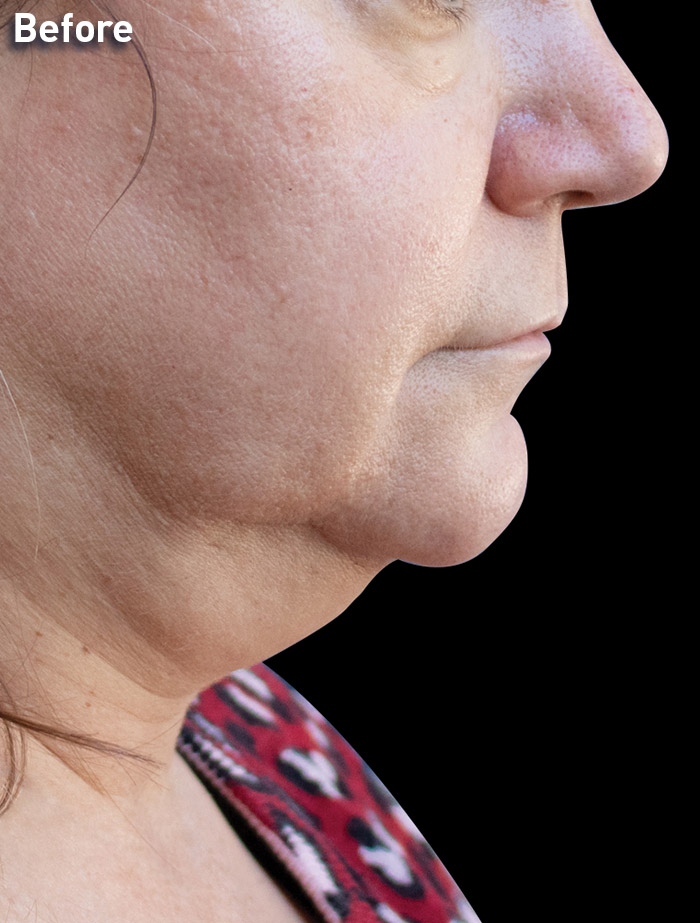
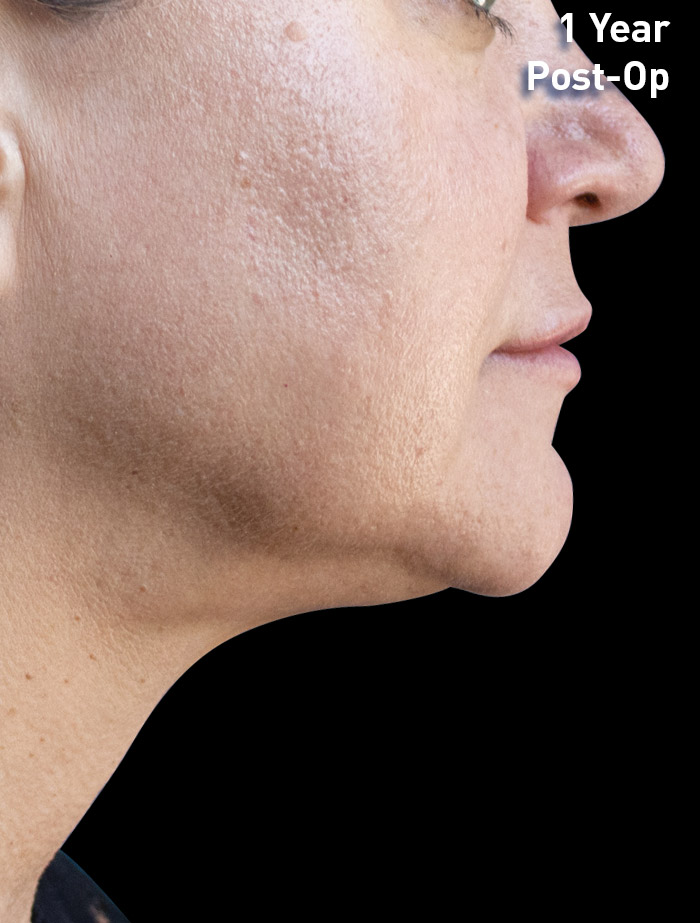
CASE 2
52-year-old female
Photos courtesy of Shazel Gharbi, MD
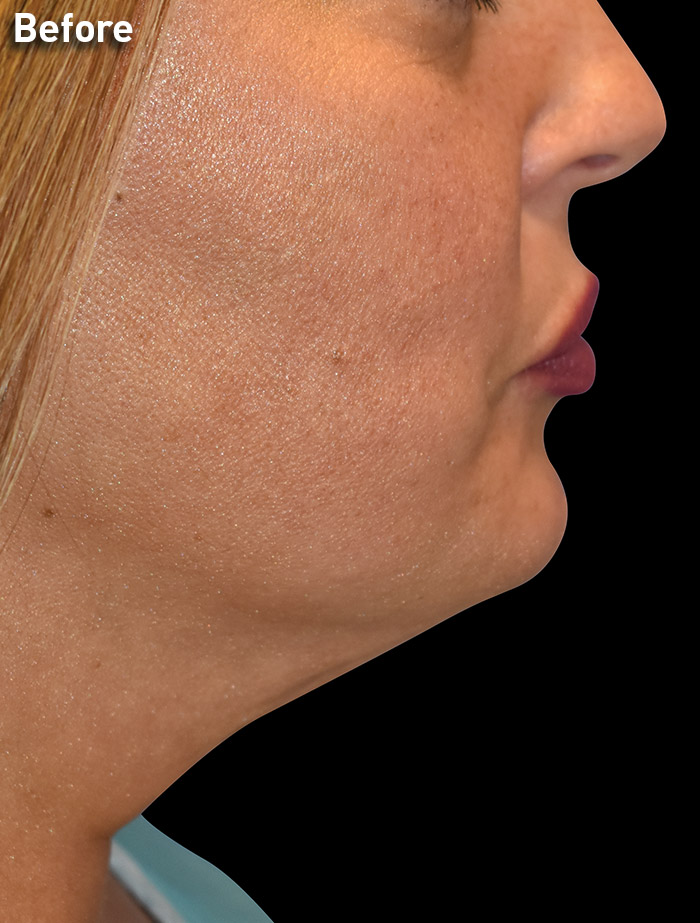
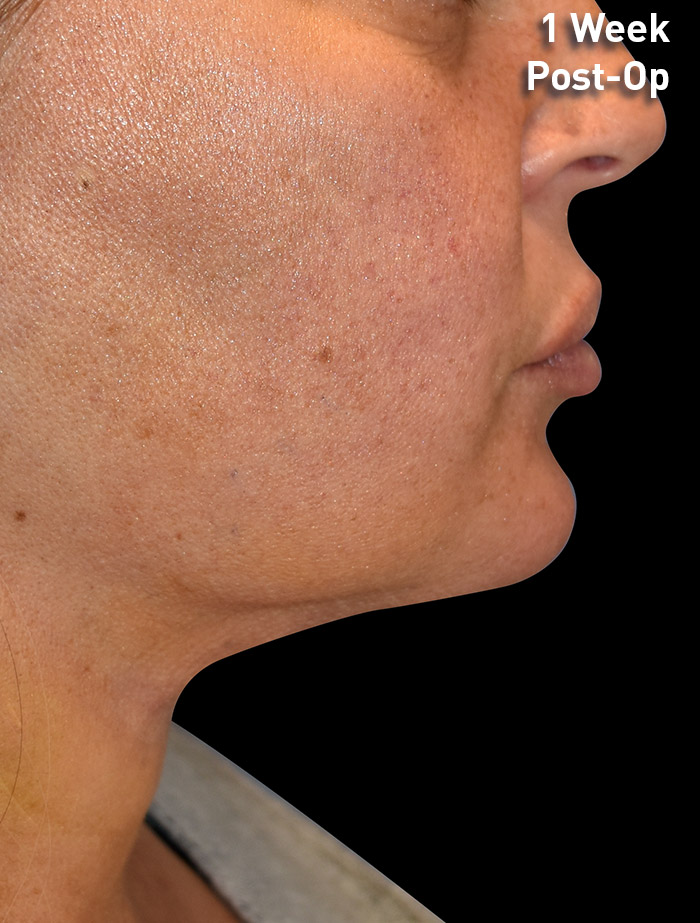
CASE 3
40-year-old female
Photos courtesy of Richard Zeff, MD
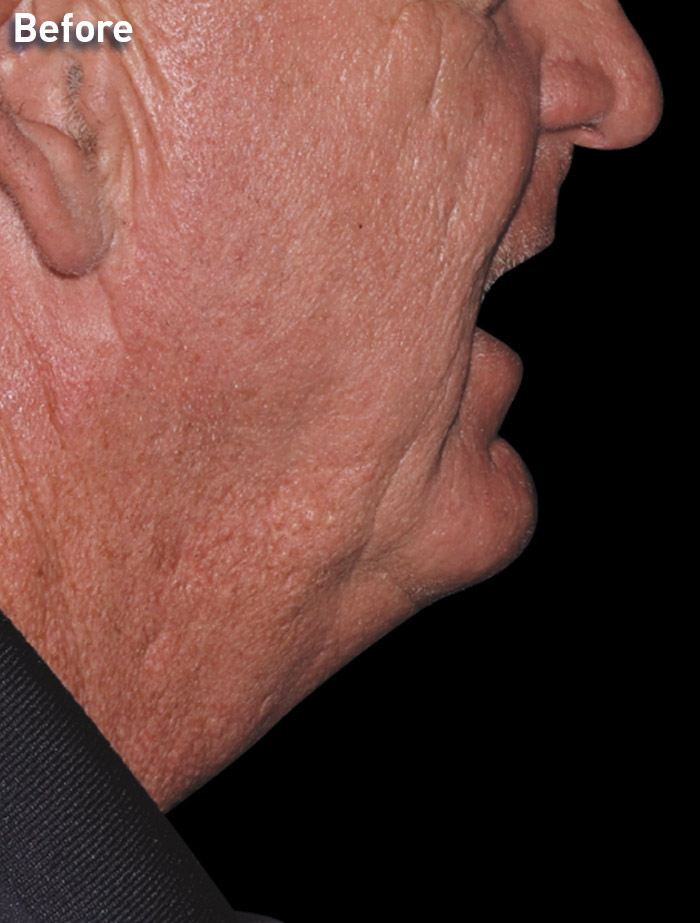
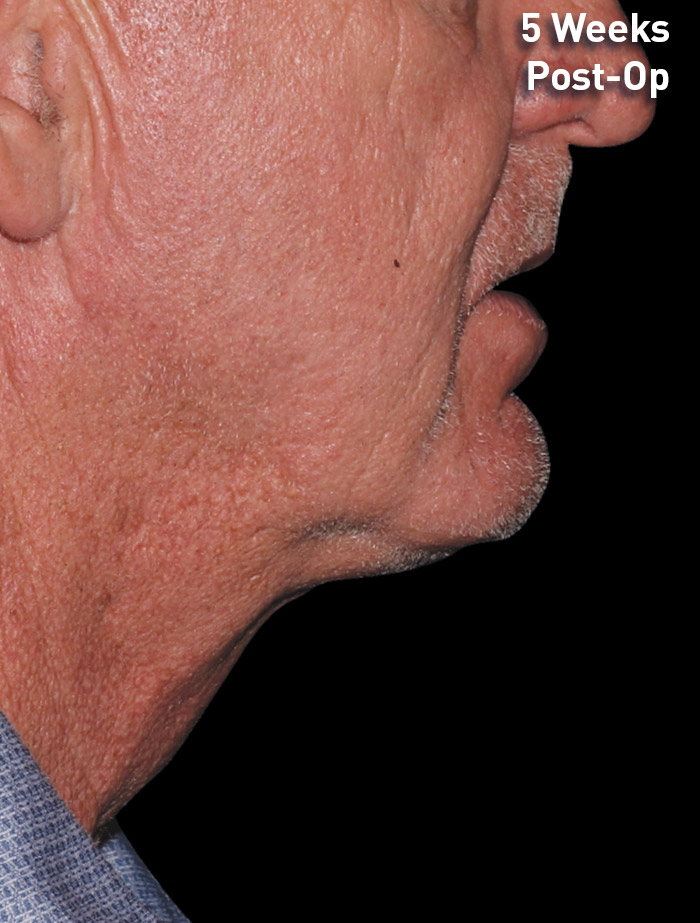
CASE 4
60-year-old male
Photos courtesy of Lisa Jenks, MD
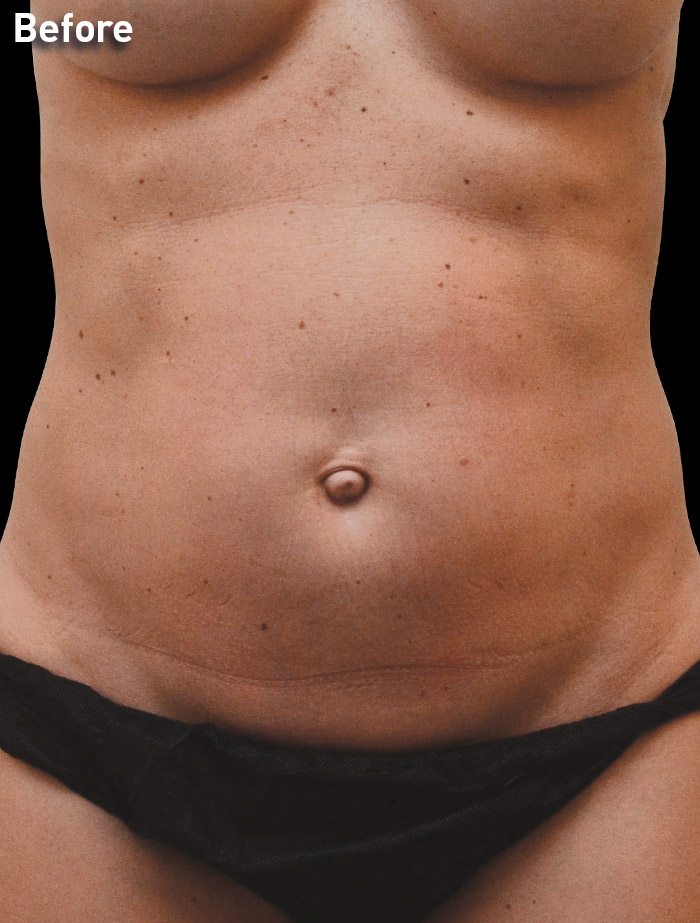
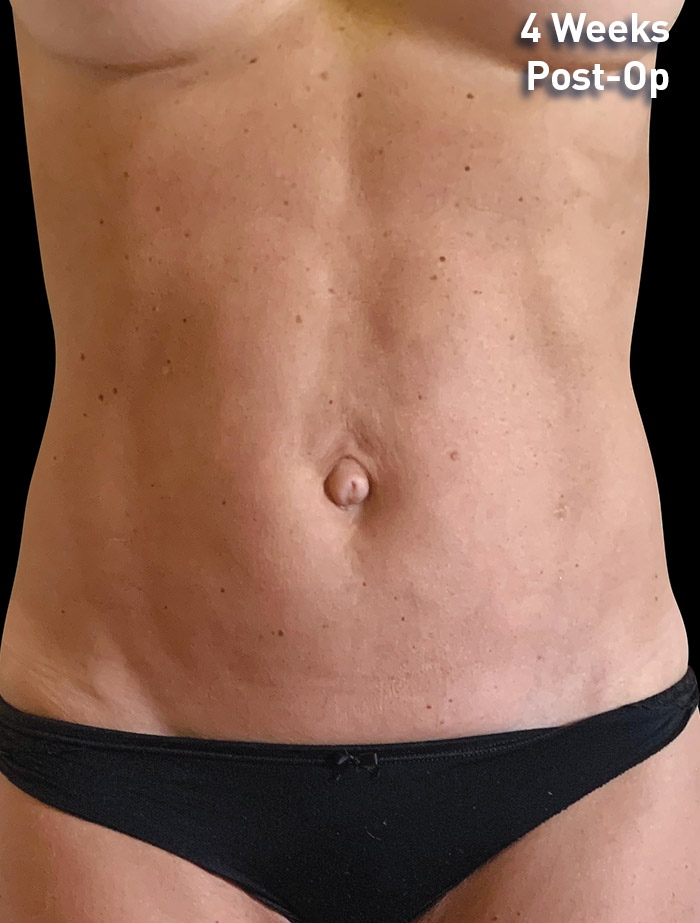
CASE 5
40-year-old female
Photos courtesy of Vaishali Doolabh, MD
*No change in weight, diet or exercise.
CASE 6
50-year-old female
Photos courtesy of Larry Fan, MD
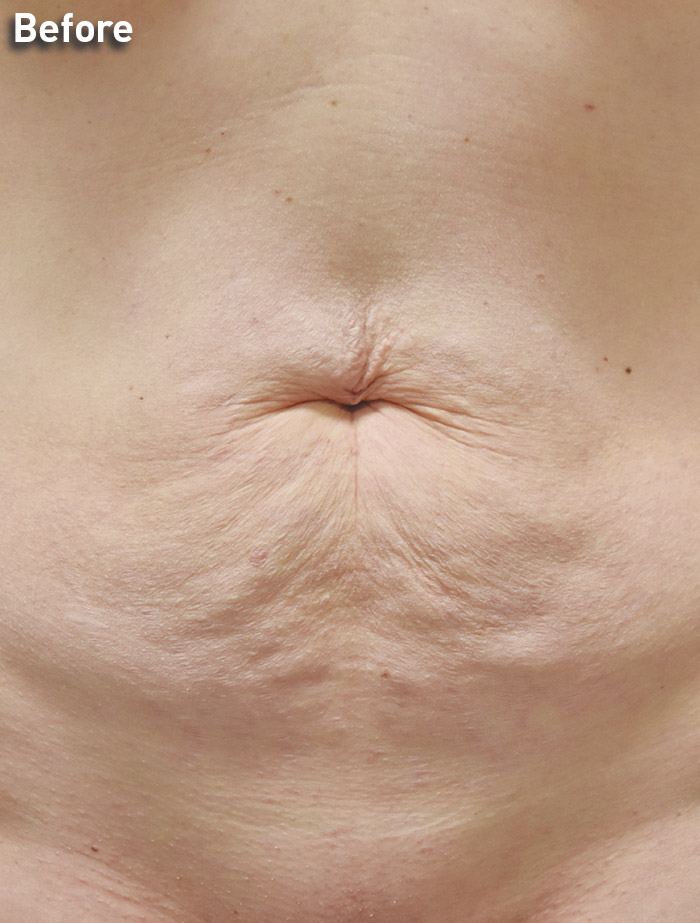
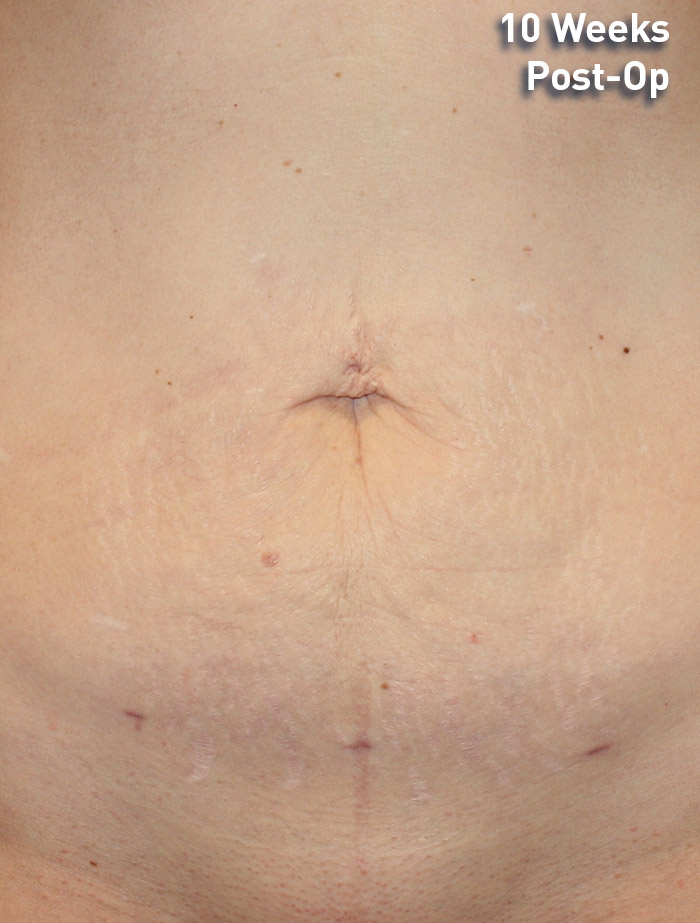
CASE 7
42-year-old female
Photos courtesy of Richard Zeff, MD
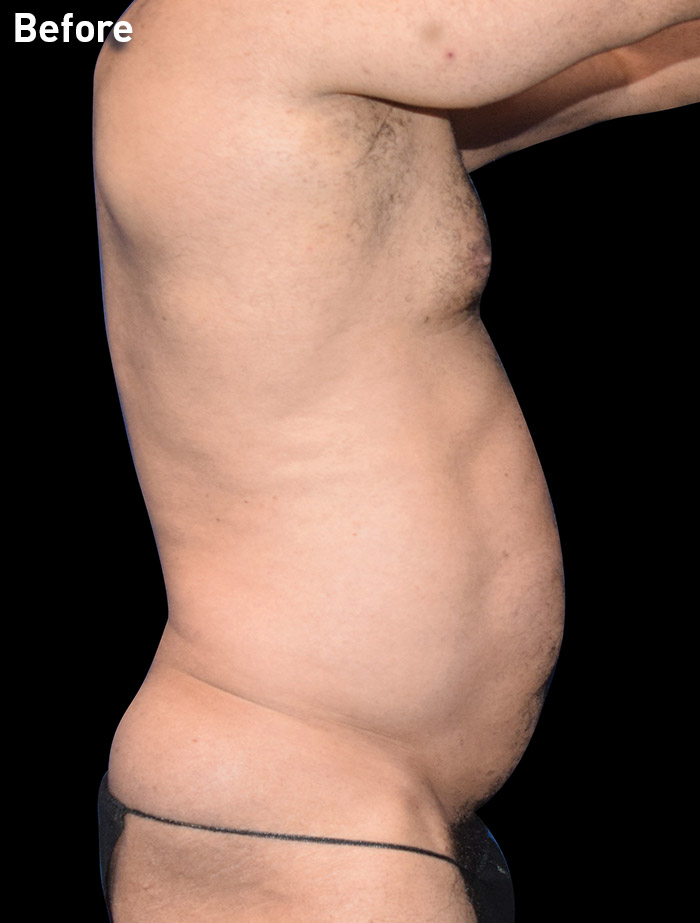
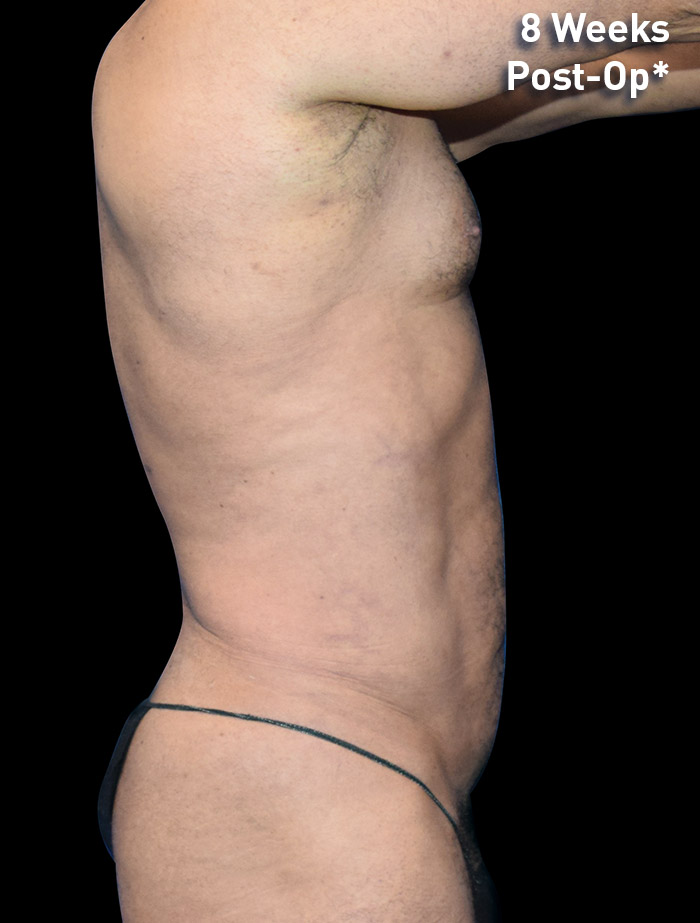
CASE 8
53-year-old male
Photos courtesy of Emil Kohan, MD
*Patient also had fat transfer to buttocks
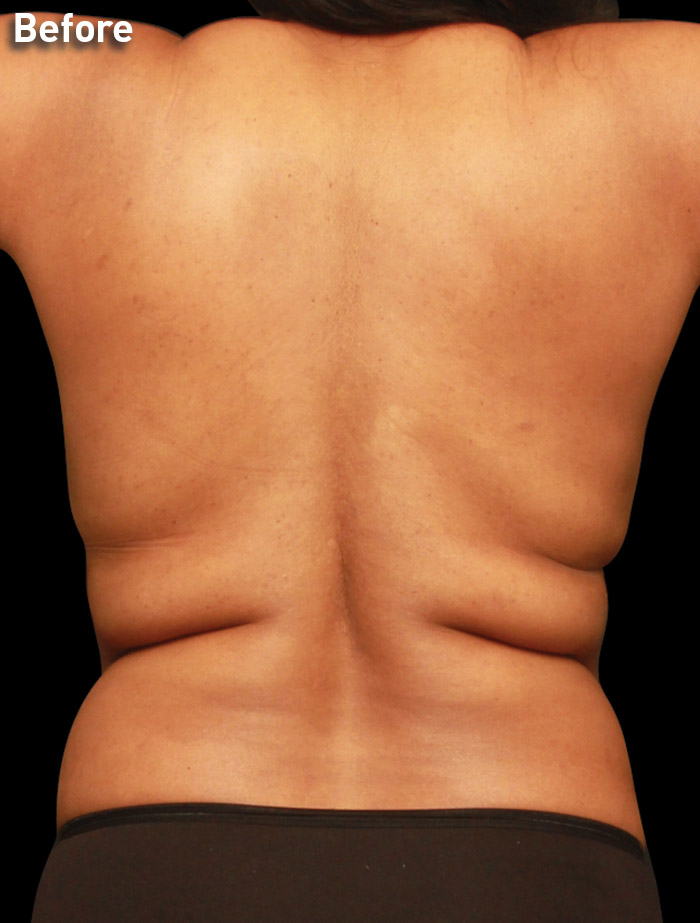
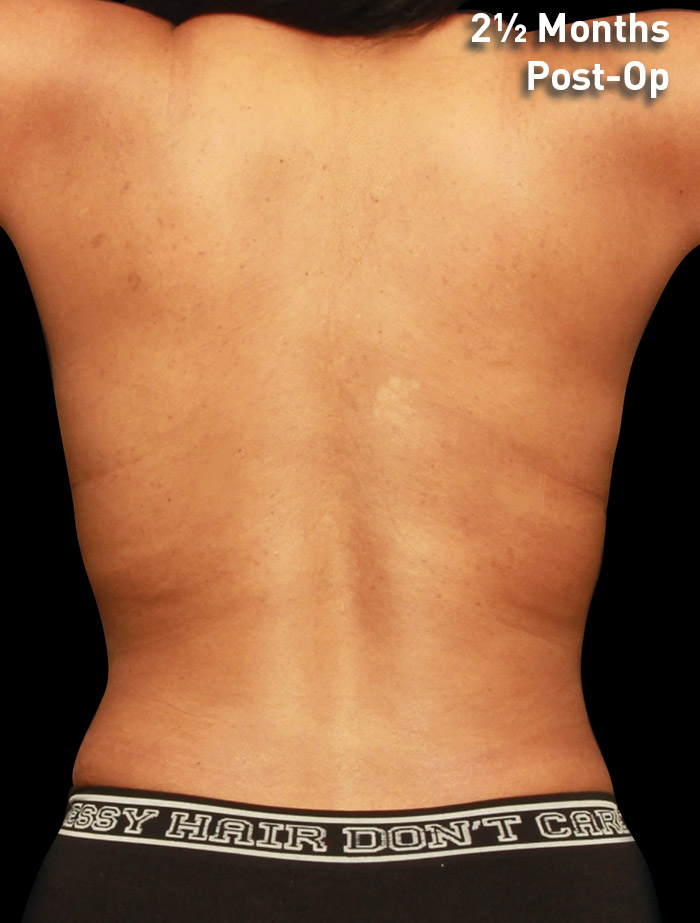
CASE 9
33-year-old female
Photos courtesy of J. Kevin Duplechain, MD
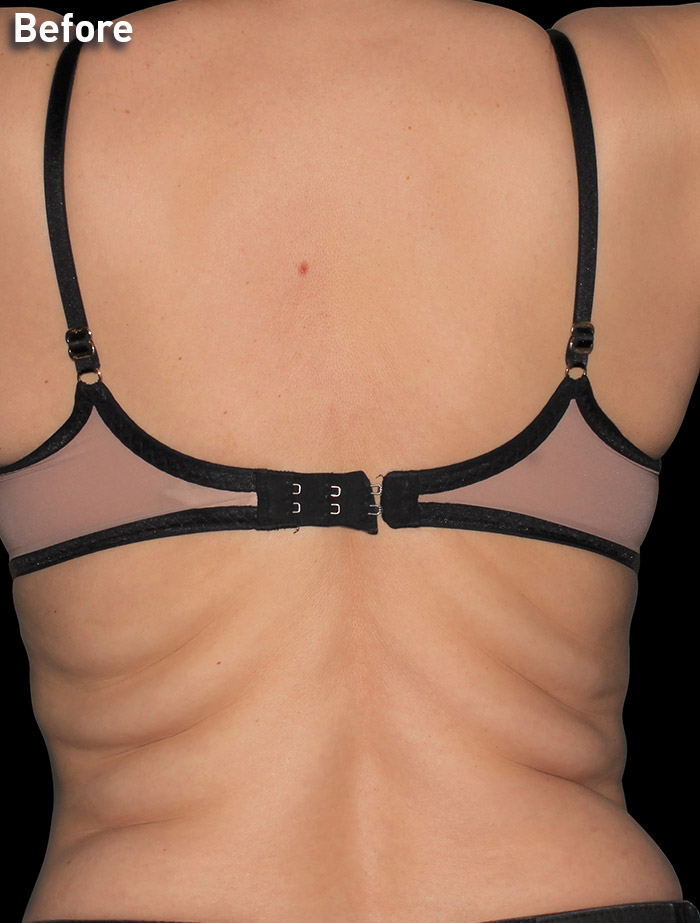
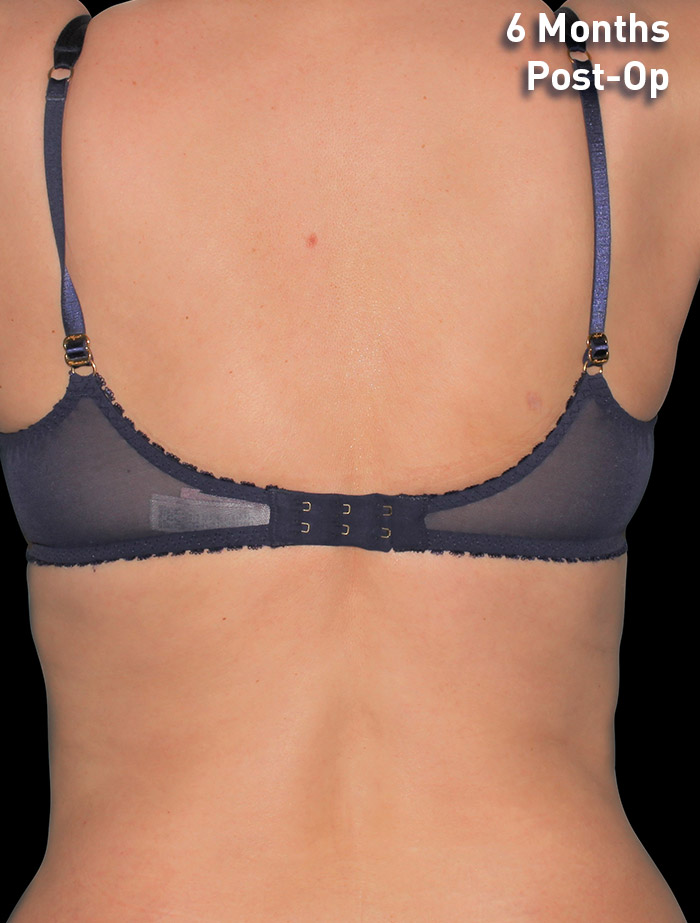
CASE 10
45-year-old female
Photos courtesy of Maria Lombardo, DO
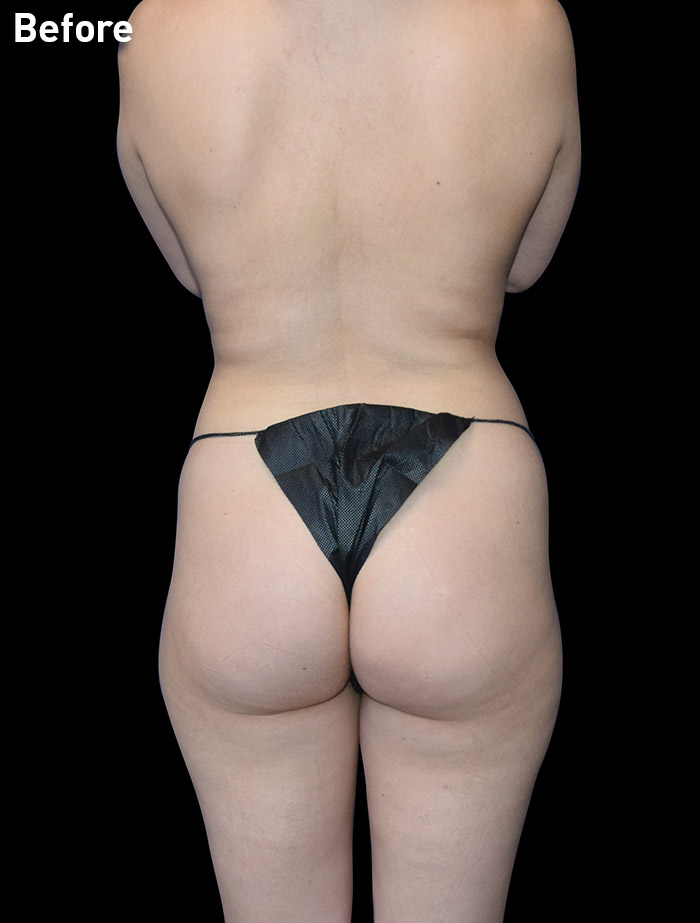
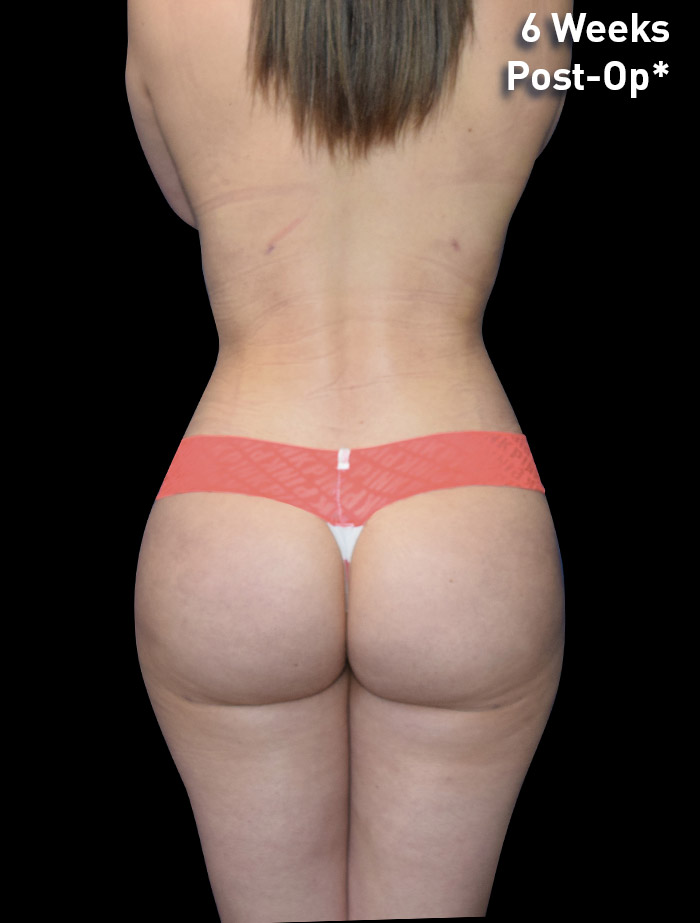
CASE 11
22-year-old female
Photos courtesy of Emil Kohan, MD
*Patient also had fat transfer to buttocks
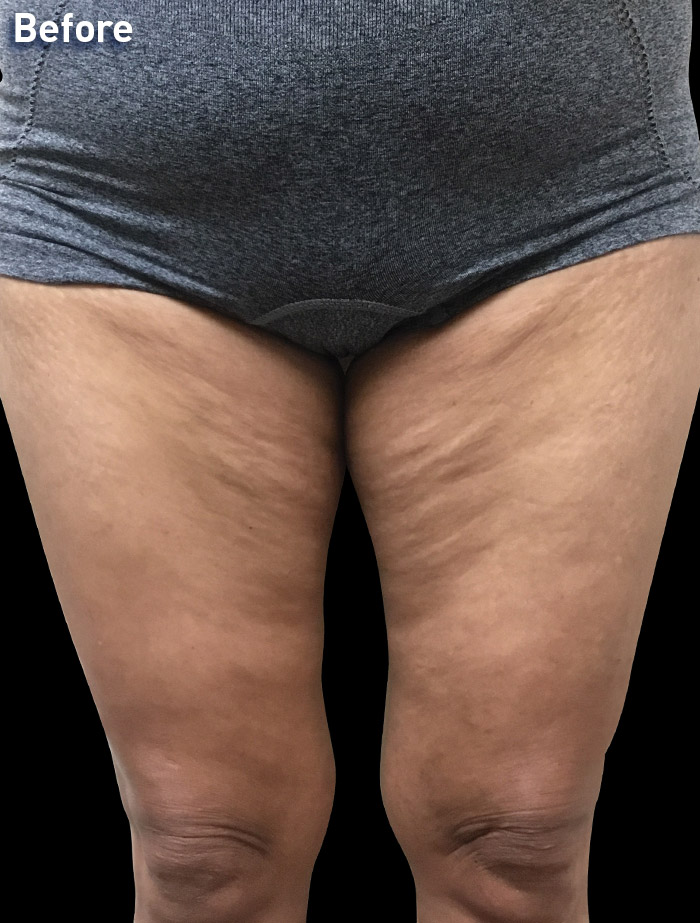
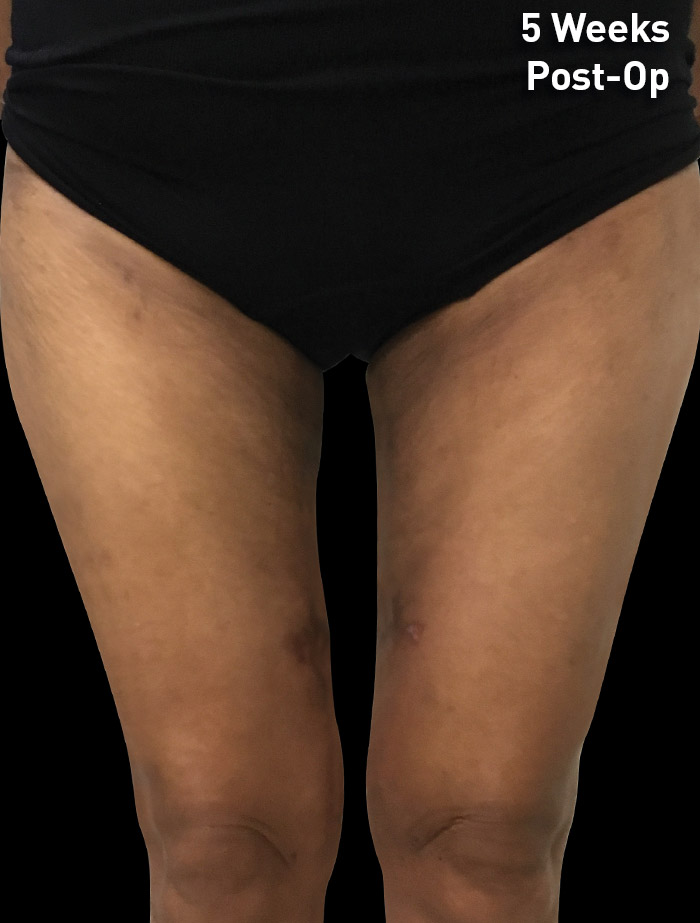
CASE 12
53-year-old female
Photos courtesy of Melinda Lacerna, MD
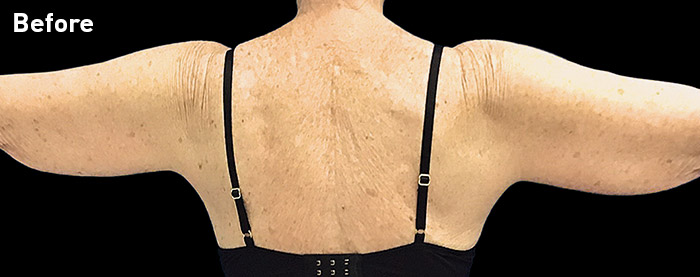
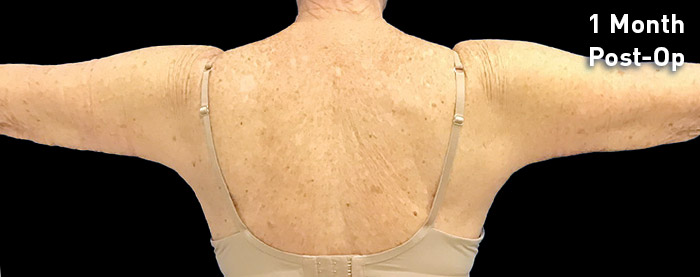
CASE 13
69-year-old female
Photos courtesy of Terry Restivo, DO


CASE 14
32-year-old female
Photos courtesy of David Melniczek, MD
LATEST NEWS
AS SEEN IN











The Renuvion APR Handpiece is intended for the delivery of radiofrequency energy and/or helium plasma where coagulation/contraction of soft tissue is needed. Soft tissue includes subcutaneous tissue. The Renuvion APR Handpiece is intended for the coagulation of subcutaneous soft tissues following liposuction for aesthetic body contouring. The Renuvion APR Handpiece is indicated for use in subcutaneous dermatological and aesthetic procedures to improve the appearance of lax (loose) skin in the neck and submental region. The Renuvion APR Handpiece is intended for the delivery of radiofrequency energy and/or helium plasma for cutting, coagulation and ablation of soft tissue during open surgical procedures. The Renuvion APR Handpiece is intended to be used with compatible electrosurgical generators owned by Apyx Medical. Not all indications are approved in all markets; check with your local sales representative for further information.
As with any aesthetic procedure, individual results may vary. As with all energy devices there are inherent risks associated with its use. Risk associated with the use of the Renuvion APR may include: helium embolism into the surgical site due to inadvertent introduction into the venous or arterial blood supply system, unintended burns (deep or superficial), pneumothorax, temporary or permanent nerve injury, ischemia, fibrosis, infection, pain, discomfort, gas buildup resulting in temporary and transient crepitus or pain, bleeding, hematoma, seroma, subcutaneous induration, pigmentation changes, increased healing time, scarring, asymmetry and/ or unacceptable cosmetic result. Please see the instructions for use for more detailed information.
Reference: 1. FDA 510(k) Premarket Notifications K230272 & K223262.
