


WHO'S BOSS


WHO'S BOSS
facial Renewal
Works Like Nothing Else
In a marketplace long-dominated by laser treatments, true innovation has been scarce. Renuvion is different. It’s the first major advancement in decades of skin treatments for the face, providing patients with dramatic and natural-looking results — This is Facial Renewal.
Beyond Resurfacing

EXPERIENCE
REAL RESULTS
In a prospective multicenter study of 55 patients1,
Renuvion demonstrated.
98.2%
TREATMENT SUCCESS
90.9%
SELF-REPORTED APPEARANCE IMPORVEMENT IN 3 MONTHS
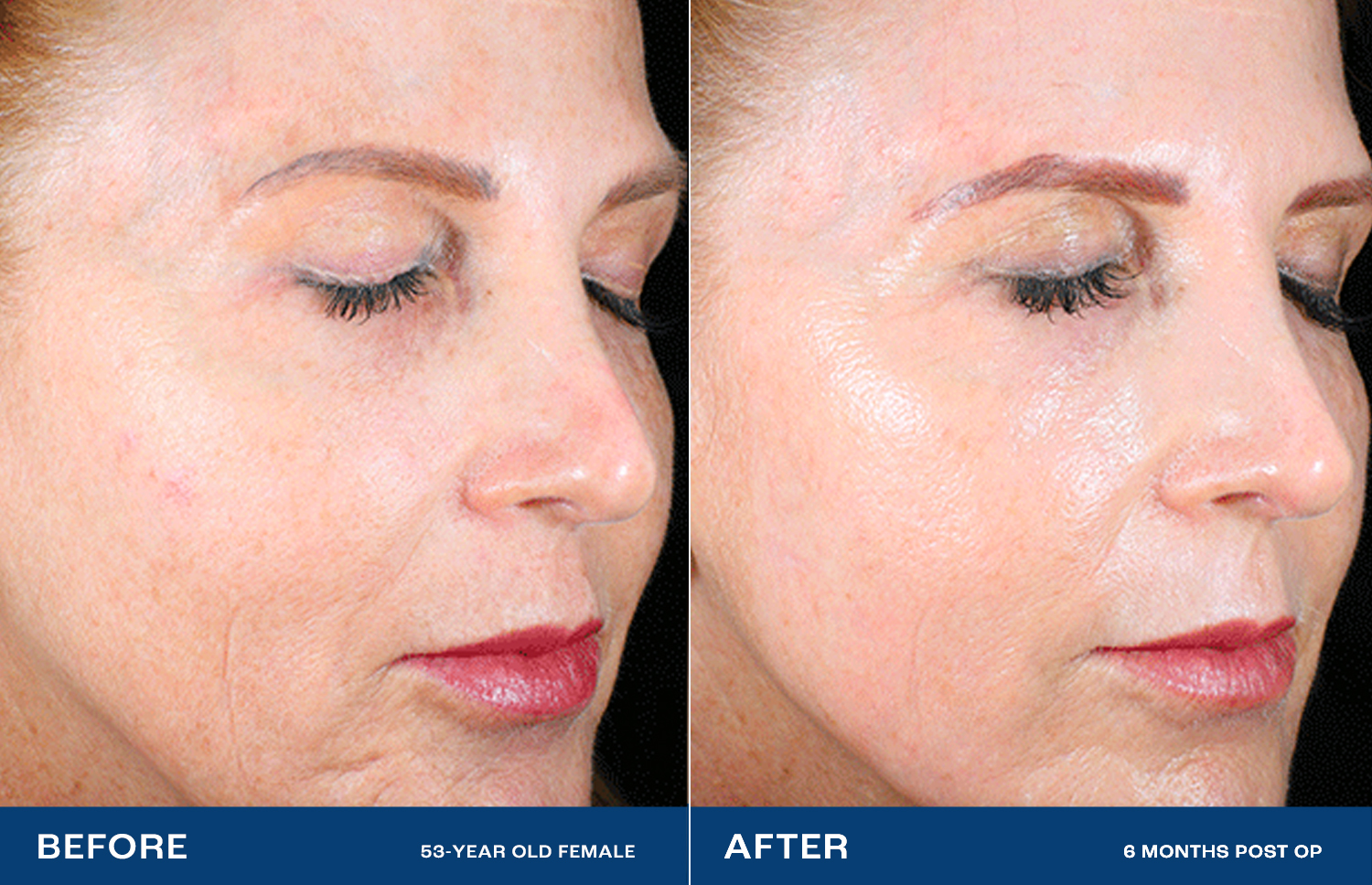
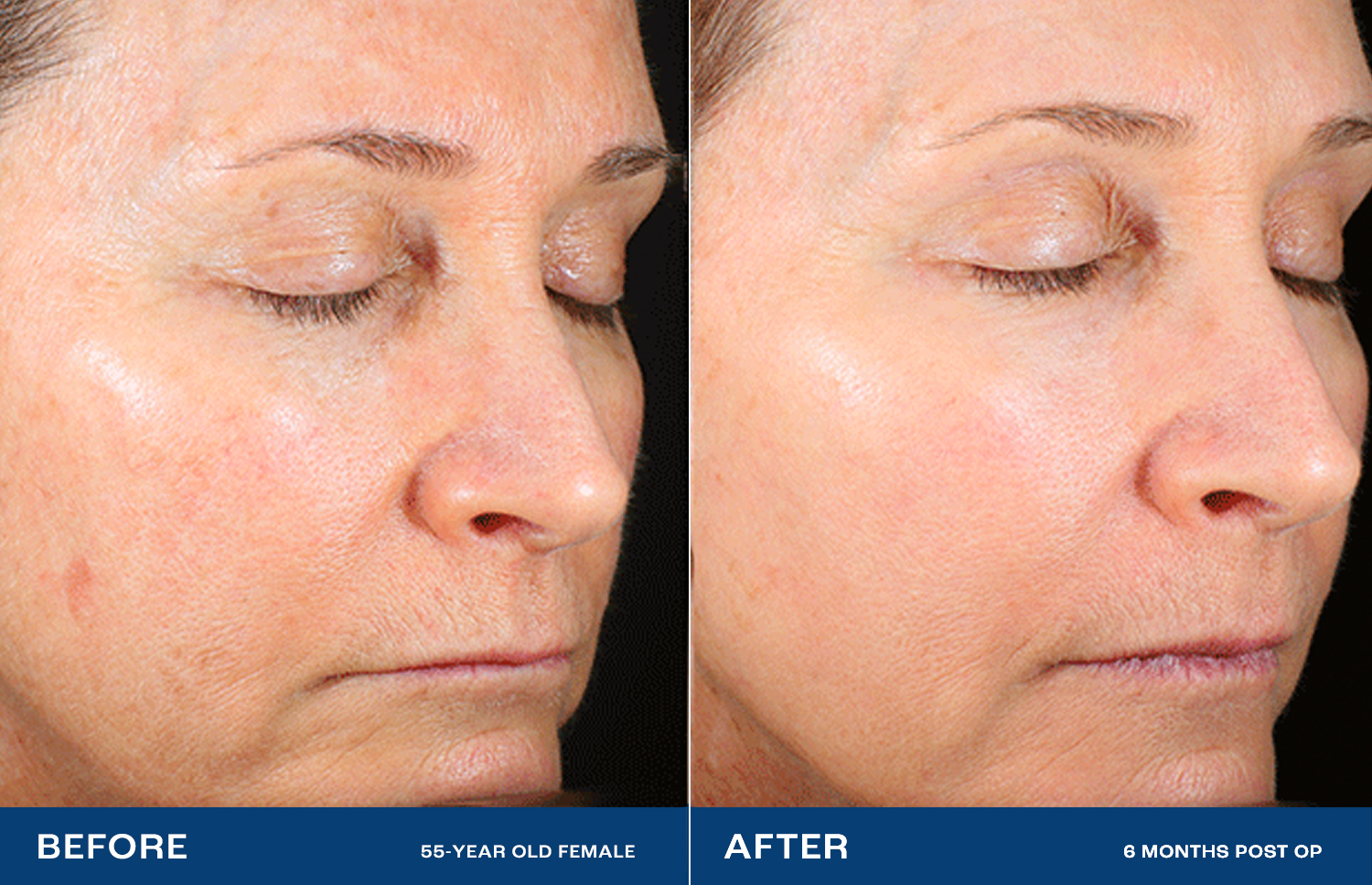
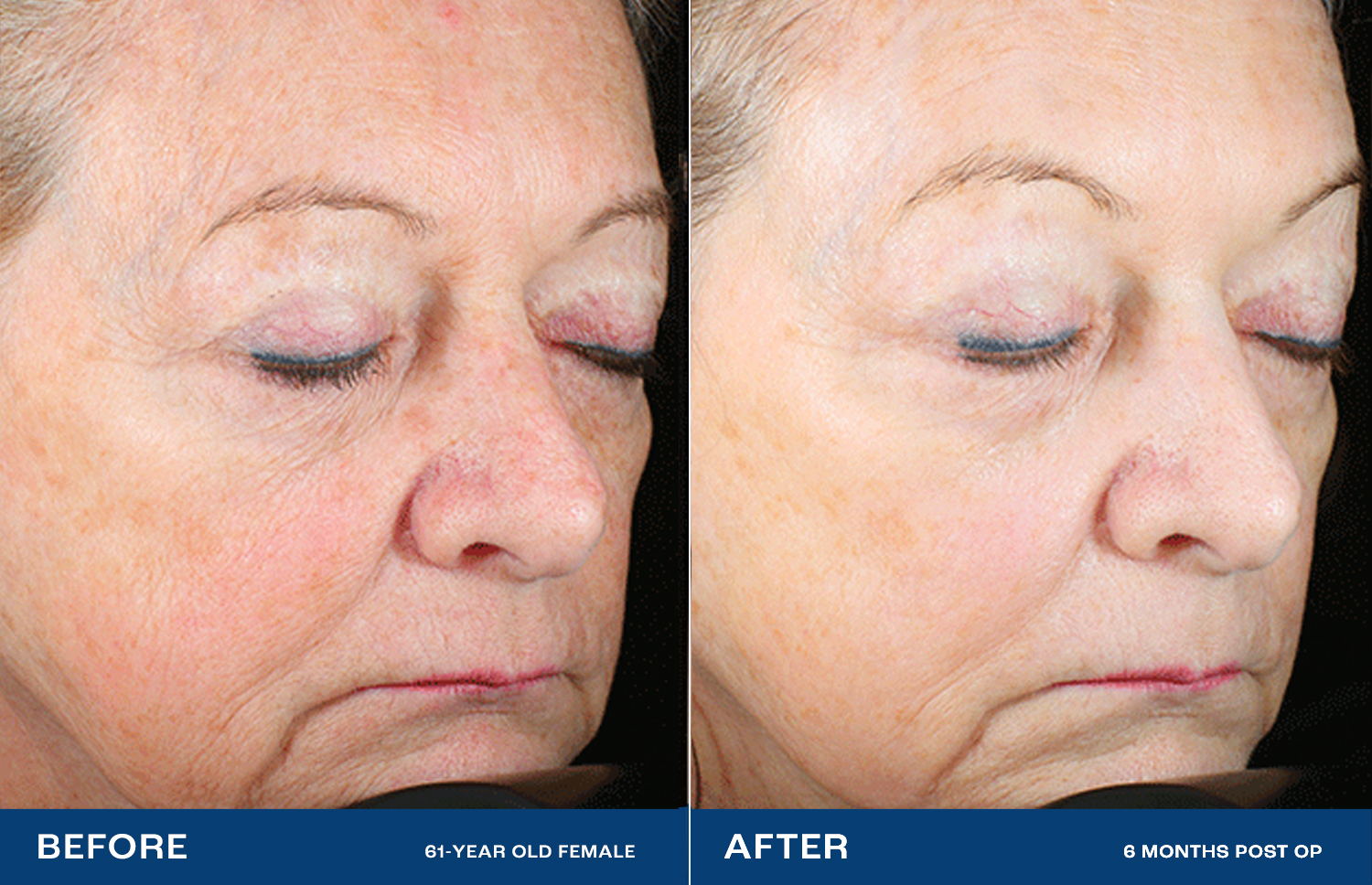
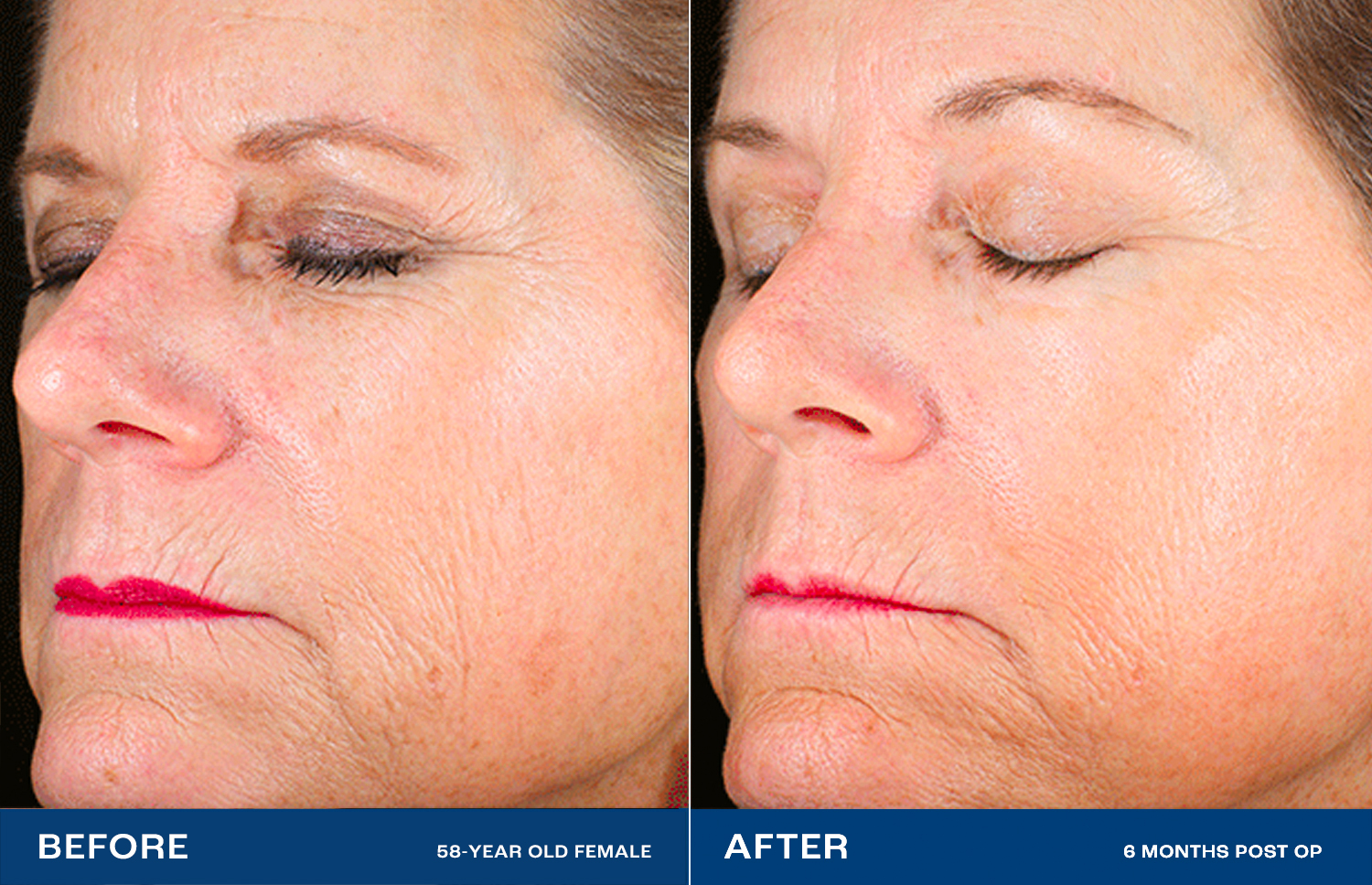

The Renuvion Difference
Unique technology, unique results
Lasers and other resurfacing technologies don’t always satisfy.2 Renuvion has been shown to dramatically reduce deep wrinkles and rhytides, with a high level of physician and patient satisfaction.1High impact – without surgery
Facelifts lift and tighten by treating the structures under the skin, but not the skin directly.3 Renuvion directly treats the skin, providing a smooth, radiant look — without cutting.1Profound change, natural effect
High-impact cosmetic procedures often have unnatural-looking results. With Renuvion, patients state that they look healthy and natural, like ‘rewinding the clock.’In preclinical testing, Renuvion showed benefits over the nitrogen plasma system, both in maximum contraction and depth of thermal effect.4
193-269%
GREATER TISSUE / WRINKLE REDUCTION
54-71%
LESS DEPTH OF THERMAL EFFECT
FREQUENTLY ASKED QUESTIONS
LATEST NEWS
AS SEEN IN











The Renuvion Dermal Handpiece is indicated for dermatological procedures for the treatment of moderate to severe wrinkles and rhytides, limited to patients with Fitzpatrick skin types I, II, or III.
Warning: Application of more than one treatment pass in the perioral area, on the forehead, and along the jawline has been associated with hypertrophic scarring.
Risks associated with the use of the Renuvion Dermal System include but are not limited to hypertrophic scarring, milia/acne, telangiectasia (spider veins), skin discoloration/hypopigmentation, dormant infection reactivation, infection, bruising or bleeding. As with any procedure, individual results may vary. As with all energy devices there are inherent risks associated with its use, refer to the IFU for further information.
References:
- Holcomb JD, Kelly M, Hamilton TK, DeLozier JB 3rd. A prospective study evaluating the use of helium plasma for dermal resurfacing. Lasers Surg Med 2020;52:940-951. https://doi.org/10.1002/lsm.23257
- Cohen SR, Henssler C, Horton K, Broder KW, Moise-Broder PA. Clinical experience with the Fraxel SR laser: 202 treatments in 59 consecutive patients. Plast Reconstr Surg 2008 May;121(5):297e-304e. doi: 10.1097/PRS.0b013e31816c3b65. PMID: 18453942.
- Shauly O, Stone GL, Shin R, Stevens GW, Gould DJ. Evaluating facelift complications and the effectiveness of the SMASectomy technique: A single center’s 15-year experience. Aesthet Surg J Open Forum 2021 Aug 20;3(4):ojab030. doi: 10.1093/asjof/ojab030. PMID: 34617012; PMCID: PMC8489308.
- Holcomb JD, Schucker A. Helium plasma skin regeneration: Evaluation of skin tissue effects in a porcine model and comparison to nitrogen plasma skin regeneration. Lasers Surg Med 2020;52:23-32. https://doi.org/10.1002/lsm.23167
