
CONTOURING TECHNOLOGY
BY SURGEONS.¹

CONTOURING TECHNOLOGY
BY SURGEONS.¹

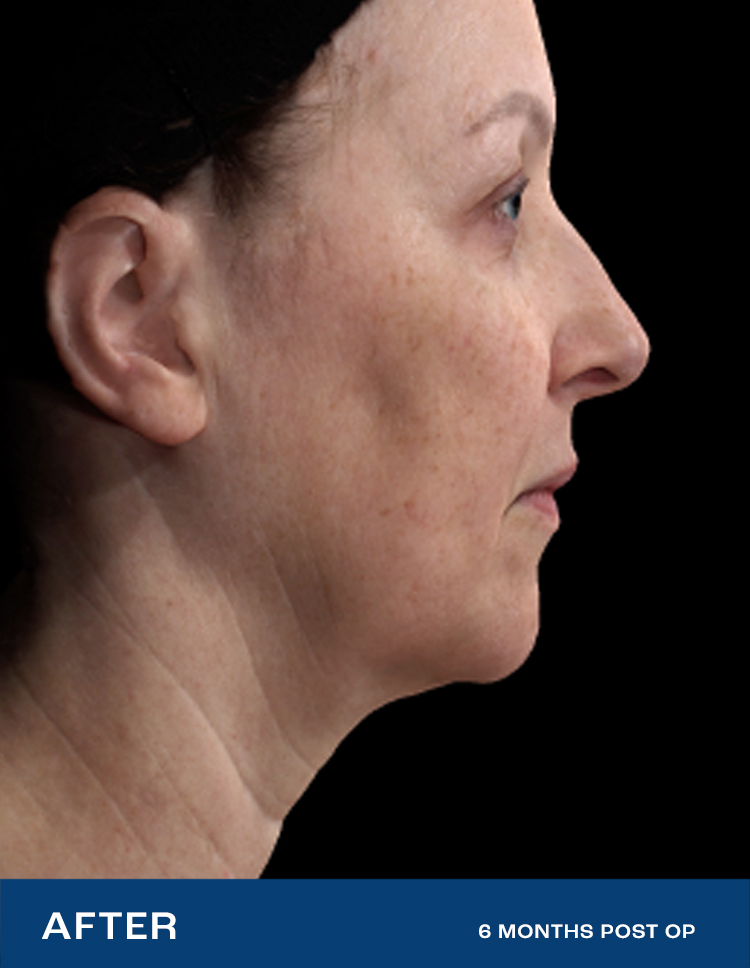
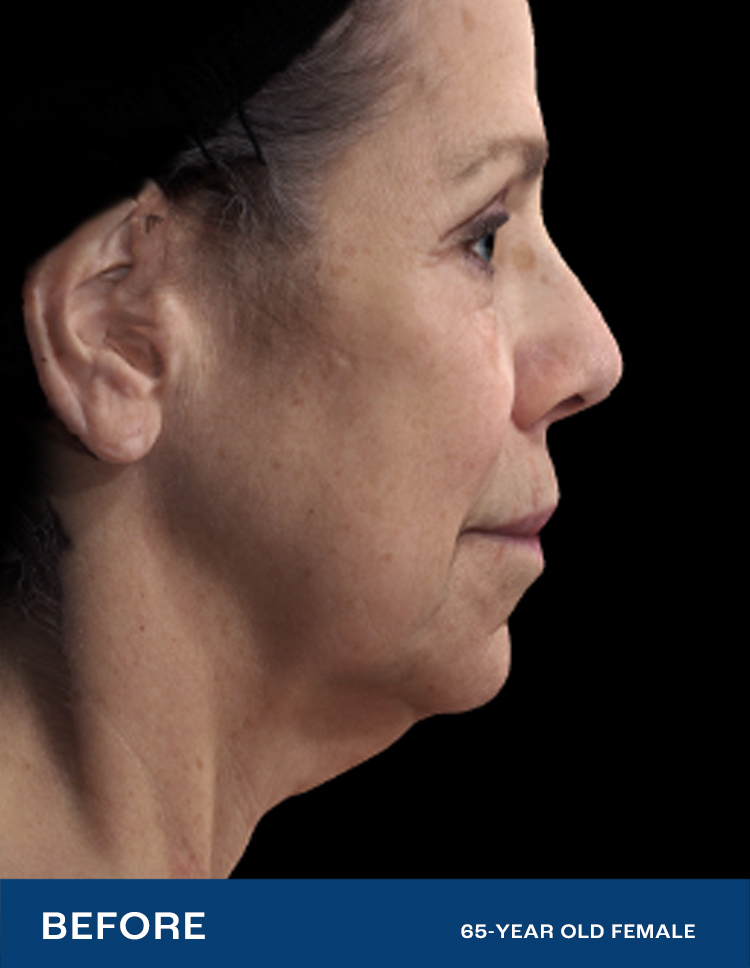
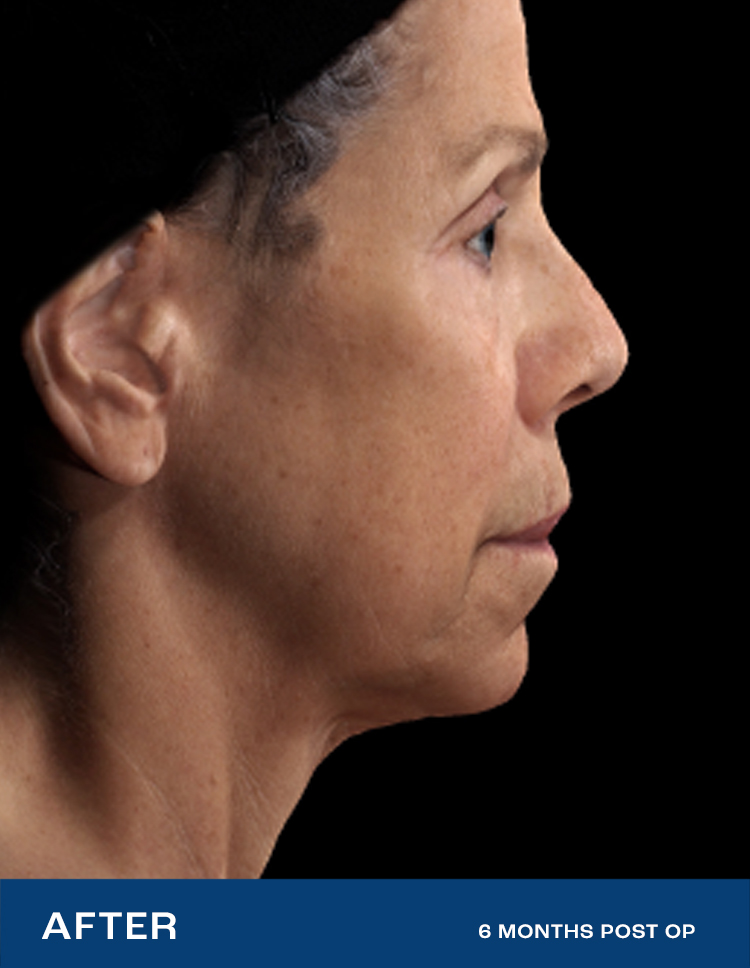
REAL RESULTS
Contouring
The Renuvion Body
Contouring
REAL RESULTS
"I had tried so many things, but my skin was still loose. After Renuvion my neck and jawline look so smooth and it feels tight like it used to. I'm so happy with the results."
- Renuvion Patient
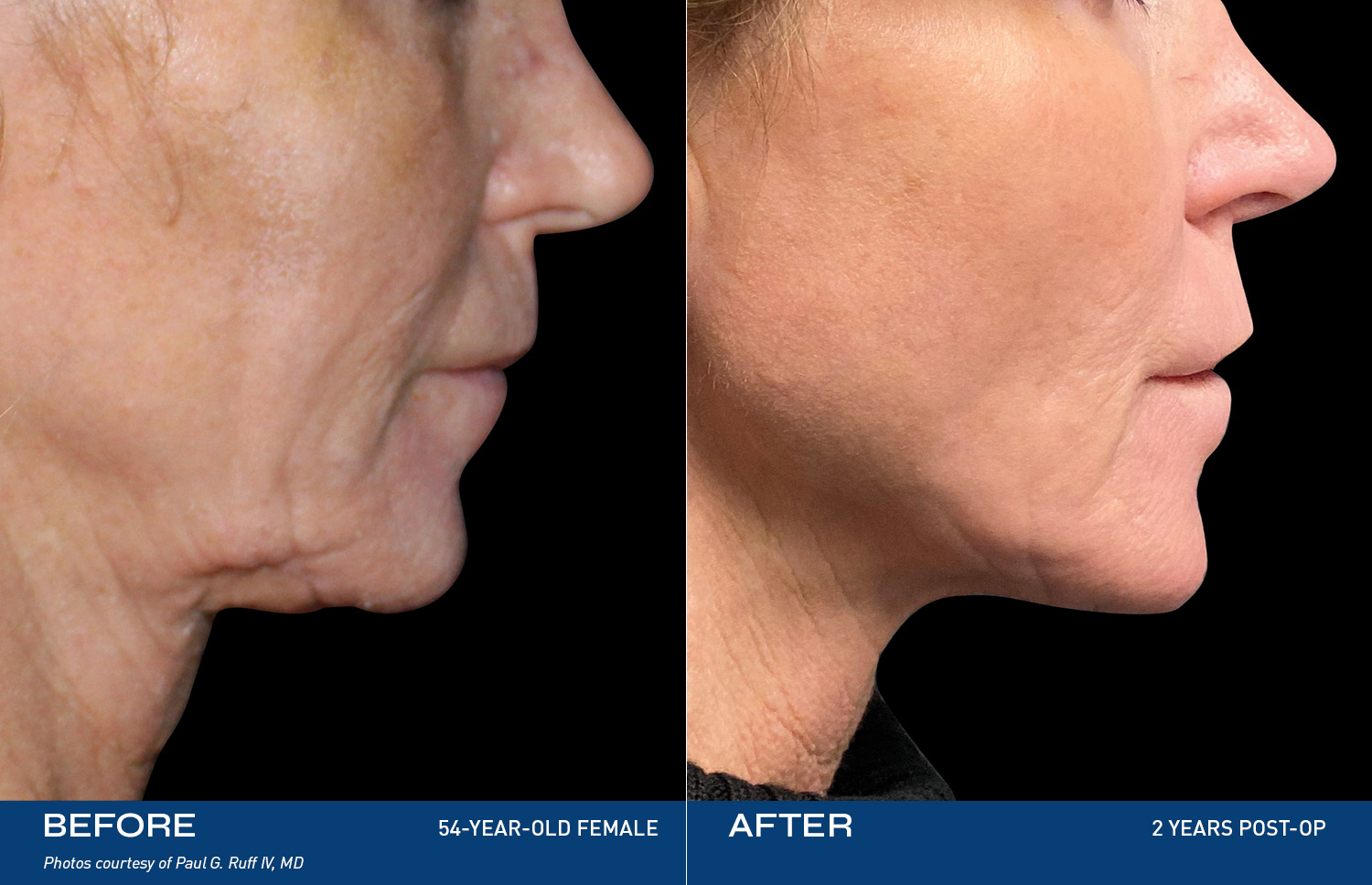
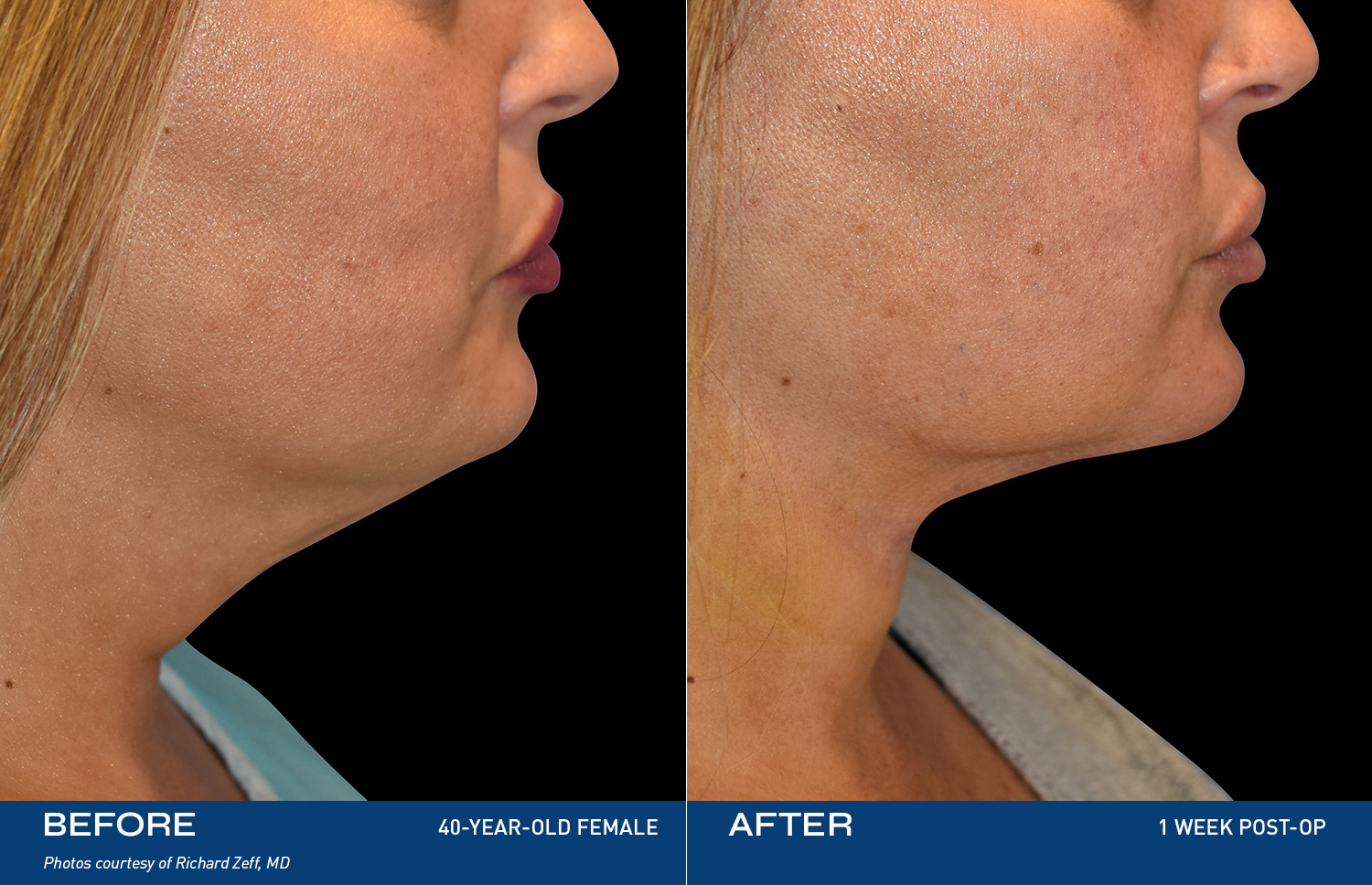
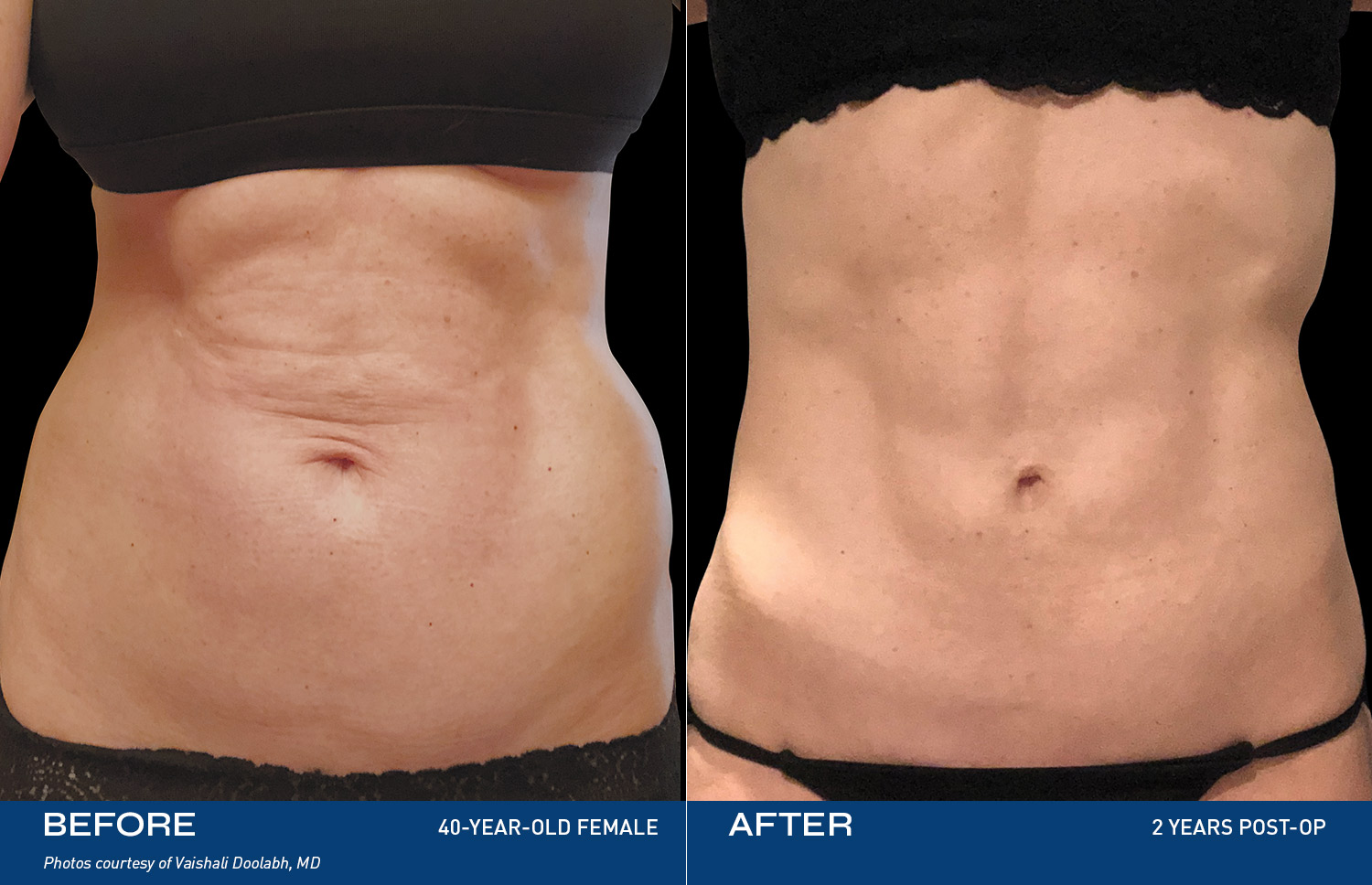
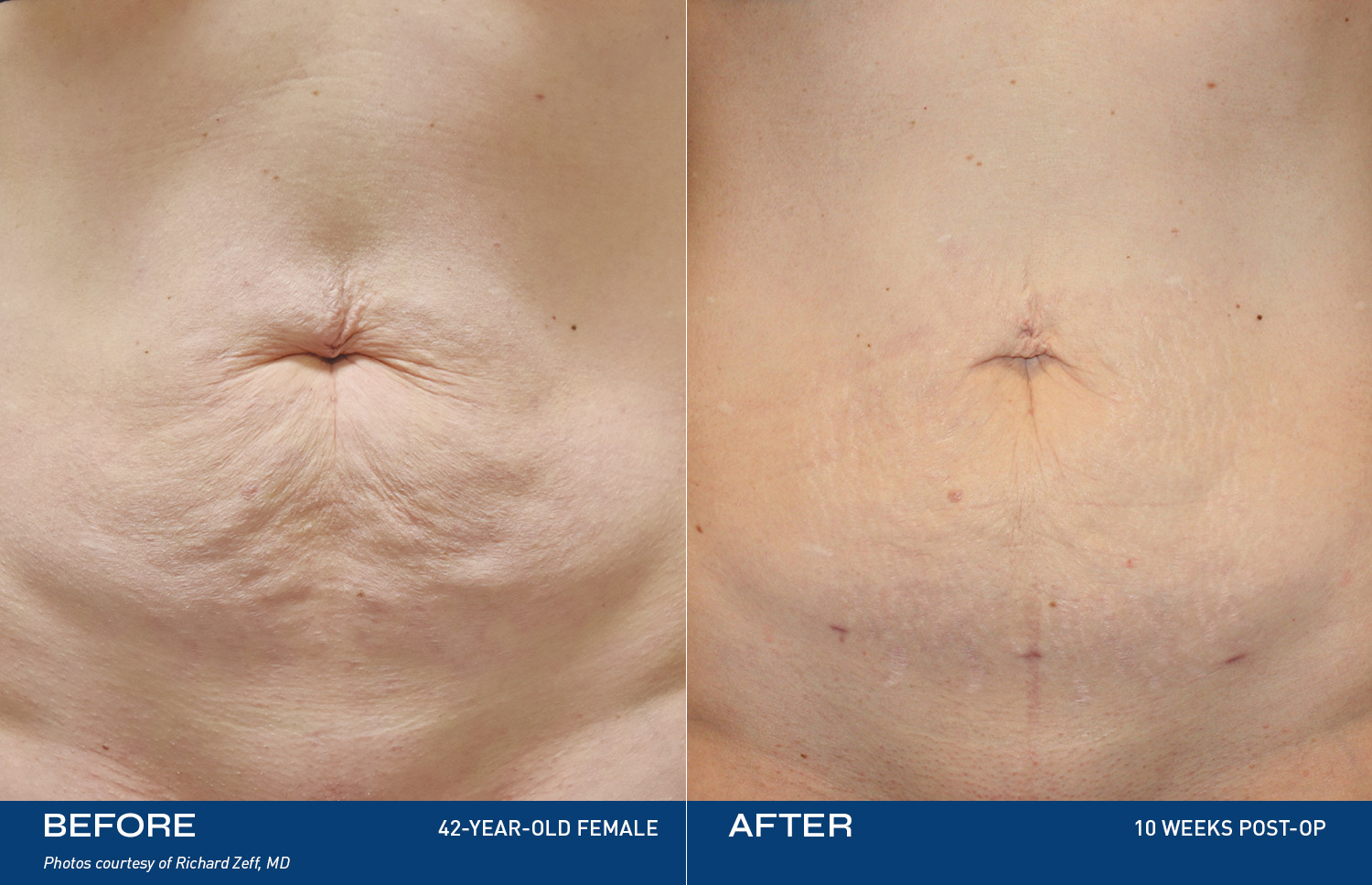
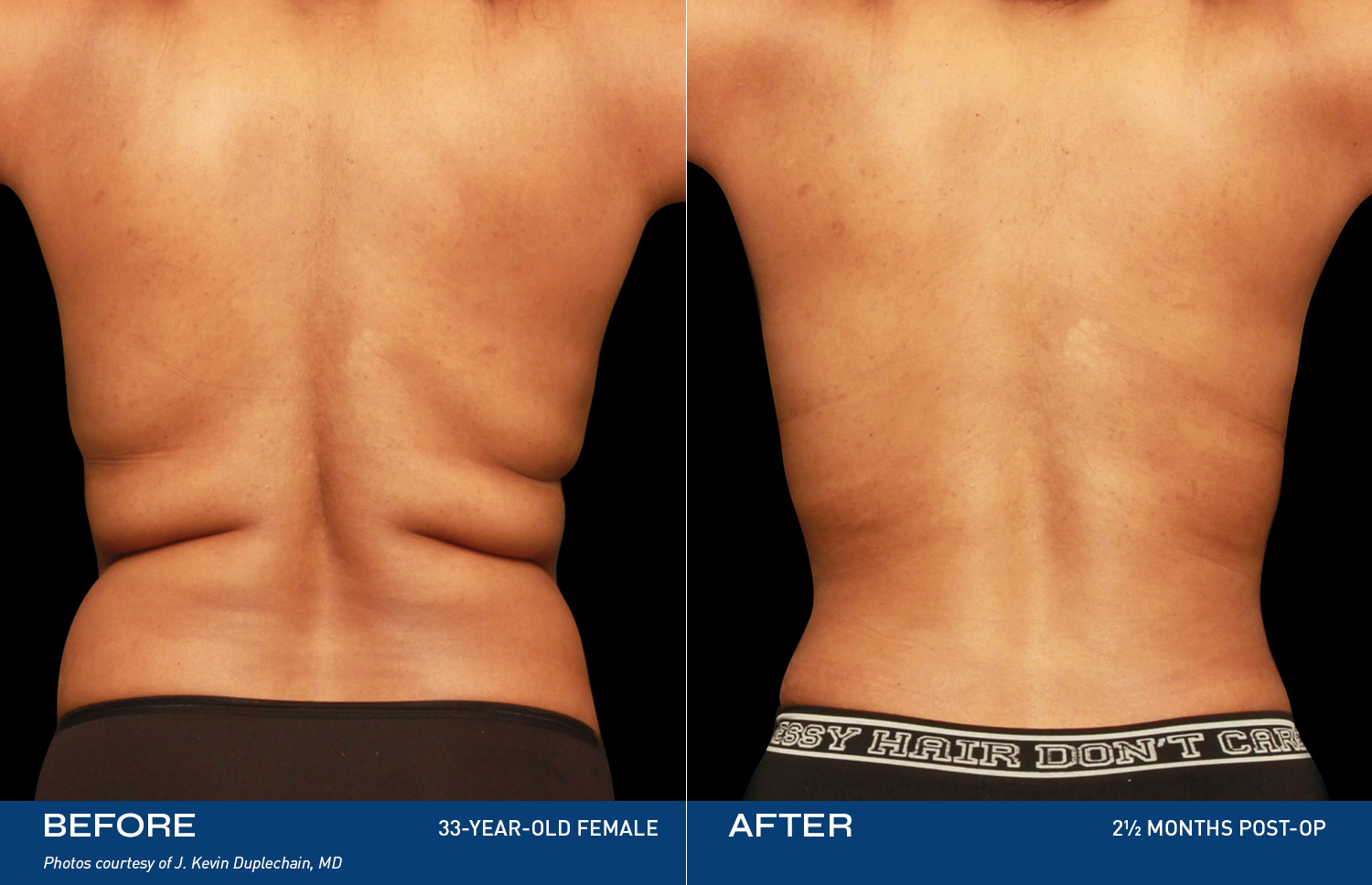
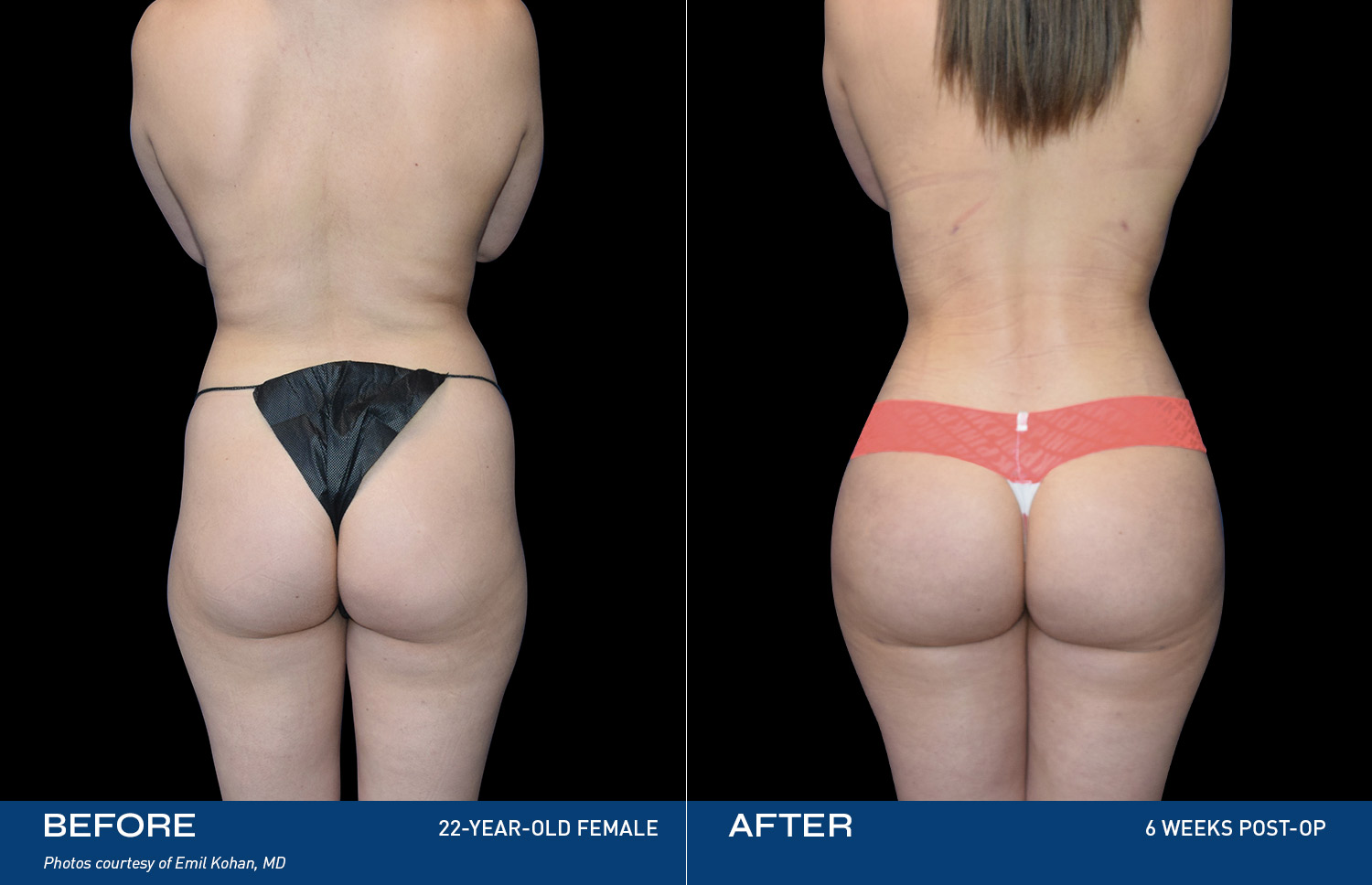
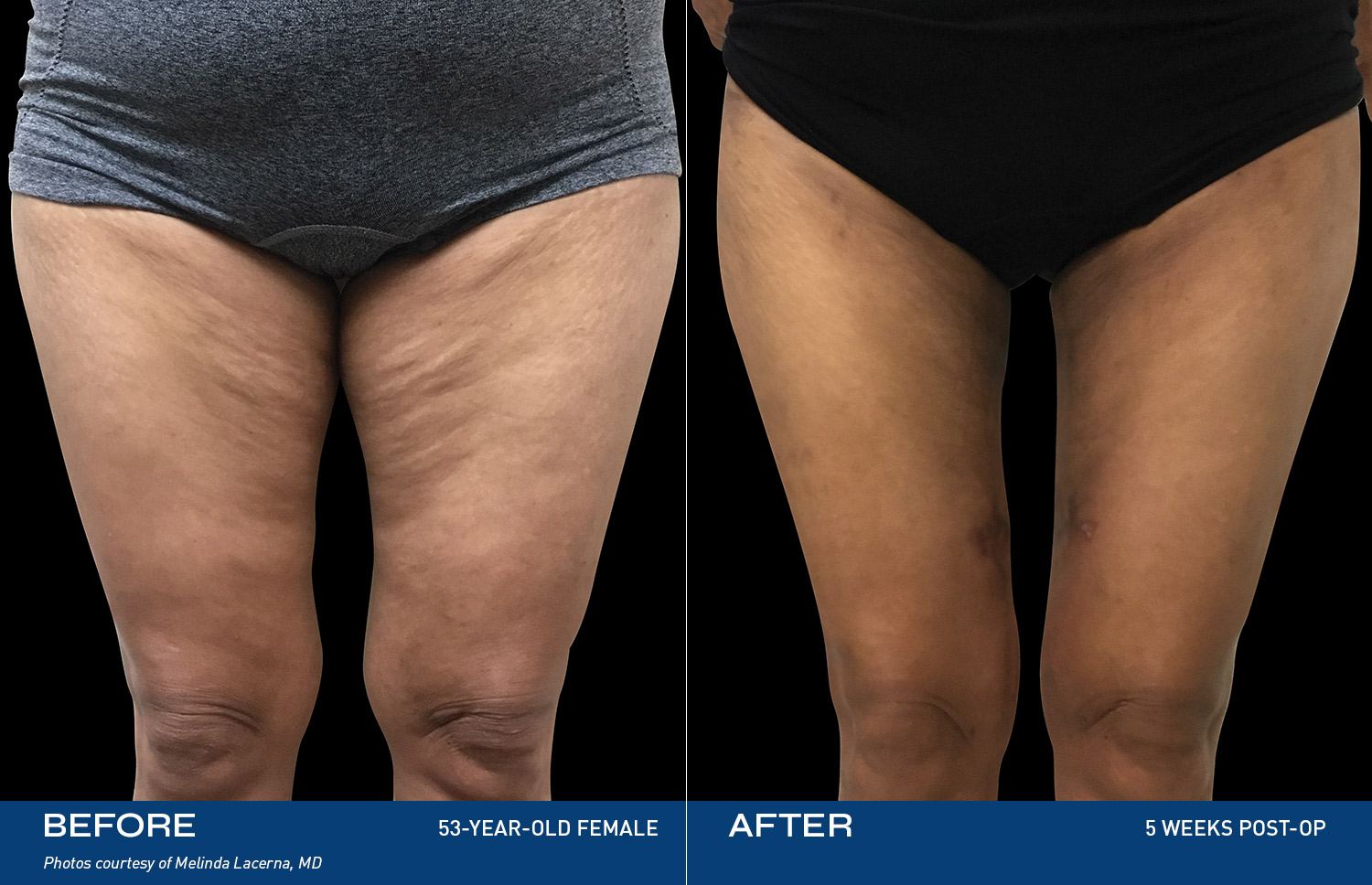
Contouring
REAL RESULTS
_____________
Results from IDE Clinical Study #G190152 where Renuvion was the only technology used.

"Over 7 years and 700 procedures later, there is still nothing that can provide the same results as RENUVION"
Joe Delozier, MD
Plastic Surgeon
Nashville, TN
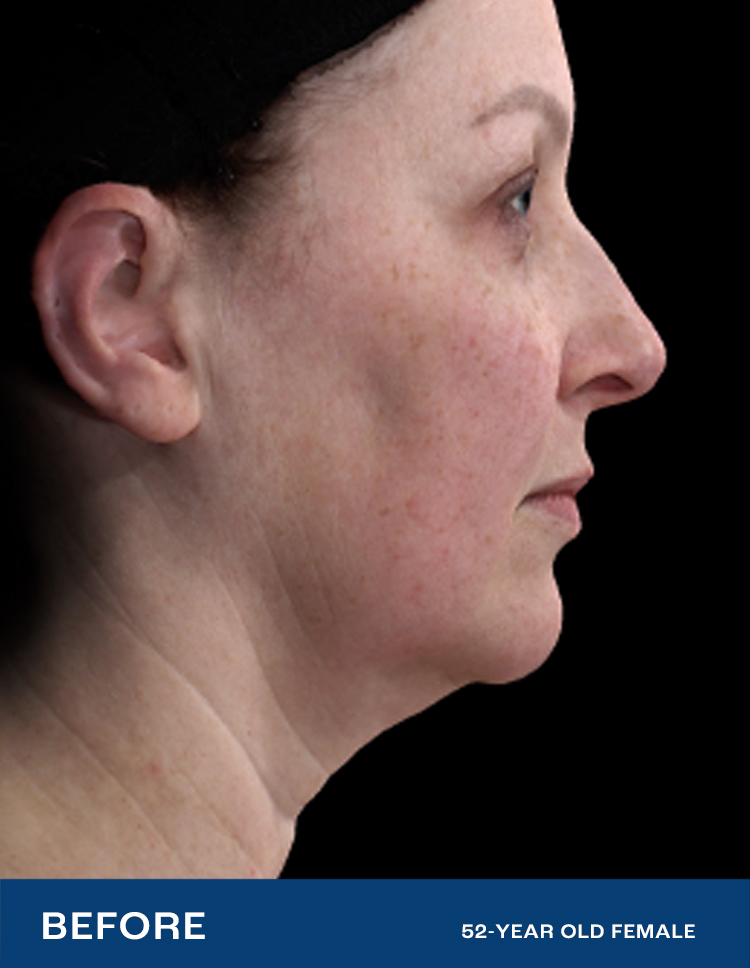
FACIAL RENEWAL
SHOW NATURE WHO'S BOSS
The first major advancement in decades of skin treatments for the face, Renuvion is a new and effective nonsurgical option for smoothing and contracting the skin — all in one procedure. This is Facial Renewal.
During the procedure, helium plasma energy is applied to a very thin layer of skin.
LATEST NEWS
AS SEEN IN











CONTOURING:
The Renuvion APR Handpiece is intended for the delivery of radiofrequency energy and/or helium plasma where coagulation/contraction of soft tissue is needed. Soft tissue includes subcutaneous tissue. The Renuvion APR Handpiece is intended for the coagulation of subcutaneous soft tissues following liposuction for aesthetic body contouring. The Renuvion APR Handpiece is indicated for use in subcutaneous dermatological and aesthetic procedures to improve the appearance of lax (loose) skin in the neck and submental region. The Renuvion APR Handpiece is intended for the delivery of radiofrequency energy and/or helium plasma for cutting, coagulation and ablation of soft tissue during open surgical procedures. The Renuvion APR Handpiece is intended to be used with compatible electrosurgical generators owned by Apyx® Medical. Not all indications are approved in all markets; check with your local sales representative for further information.
As with any aesthetic procedure, individual results may vary. As with all energy devices there are inherent risks associated with its use. Risk associated with the use of the Renuvion APR may include: helium embolism into the surgical site due to inadvertent introduction into the venous or arterial blood supply system, unintended burns (deep or superficial), pneumothorax, temporary or permanent nerve injury, ischemia, fibrosis, infection, pain, discomfort, gas buildup resulting in temporary and transient crepitus or pain, bleeding, hematoma, seroma, subcutaneous induration, pigmentation changes, increased healing time, scarring, asymmetry and/ or unacceptable cosmetic result. Please see the instructions for use for more detailed information.
FACIAL RENEWAL:
The Renuvion Dermal Handpiece is indicated for dermatological procedures for the treatment of moderate to severe wrinkles and rhytides, limited to patients with Fitzpatrick skin types I, II, or III.
Warning: Application of more than one treatment pass in the perioral area, on the forehead, and along the jawline has been associated with hypertrophic scarring.
Risks associated with the use of the Renuvion Dermal System include but are not limited to hypertrophic scarring, milia/acne, telangiectasia (spider veins), skin discoloration/hypopigmentation, dormant infection reactivation, infection, bruising or bleeding. As with any procedure, individual results may vary. As with all energy devices there are inherent risks associated with its use, refer to the IFU for further information.
Reference:
1. 4 out of 5 surgeons agree Renuvion is the #1 trusted body contouring technology. Results based on a 2024 Renuvion Physician survey conducted by Wakefield Research. Data on File.
2. FDA 510(k) Premarket Notifications K230272 & K223262.


